What Is The Electron Configuration For Neon
Muz Play
Mar 25, 2025 · 5 min read
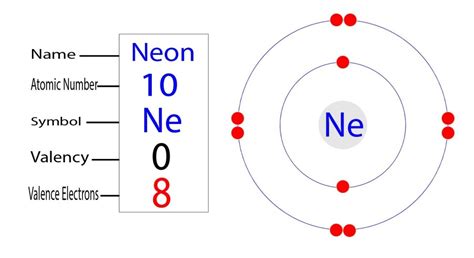
Table of Contents
What is the Electron Configuration for Neon? A Deep Dive into Atomic Structure
Neon, the tenth element on the periodic table, is a noble gas known for its inert nature and vibrant red-orange glow in discharge tubes. Understanding its electron configuration is key to grasping its chemical behavior and properties. This comprehensive guide will delve into the electron configuration of neon, exploring its underlying principles and implications.
Understanding Electron Configuration
Before we dive into neon's specific configuration, let's establish a foundational understanding of what electron configuration means. An electron configuration describes the arrangement of electrons in an atom's electron shells and subshells. It dictates an atom's chemical properties, its reactivity, and its behavior in various environments. This arrangement follows specific rules governed by quantum mechanics.
The Aufbau Principle and Hund's Rule
Two crucial principles govern electron configuration:
-
The Aufbau Principle: This principle states that electrons first fill the lowest energy levels available before occupying higher energy levels. Think of it like filling a building from the ground floor up—you wouldn't start on the tenth floor!
-
Hund's Rule: This rule states that electrons will individually occupy each orbital within a subshell before doubling up in any single orbital. Each orbital can hold a maximum of two electrons, and these electrons must have opposite spins (represented by arrows pointing up and down). Imagine it like filling seats on a bus—each person gets their own seat before anyone doubles up.
Electron Shells and Subshells
Electrons reside in shells, denoted by principal quantum numbers (n = 1, 2, 3, etc.). Each shell contains subshells, designated by letters: s, p, d, and f. Each subshell has a specific number of orbitals:
- s subshell: Contains 1 orbital, holding a maximum of 2 electrons.
- p subshell: Contains 3 orbitals, holding a maximum of 6 electrons.
- d subshell: Contains 5 orbitals, holding a maximum of 10 electrons.
- f subshell: Contains 7 orbitals, holding a maximum of 14 electrons.
Neon's Electron Configuration: 1s²2s²2p⁶
Now, let's apply these principles to neon (Ne), which has an atomic number of 10. This means it has 10 protons in its nucleus and therefore 10 electrons surrounding it. Following the Aufbau principle and Hund's rule, the electron configuration for neon is: 1s²2s²2p⁶.
Let's break this down:
-
1s²: The first shell (n=1) contains the 's' subshell, which holds two electrons. The superscript '2' indicates that both electrons are present.
-
2s²: The second shell (n=2) also contains an 's' subshell, which accommodates another two electrons.
-
2p⁶: The second shell also contains a 'p' subshell. This subshell can hold up to six electrons, and in neon, all three orbitals are filled with electron pairs.
This complete filling of the second shell is crucial to understanding neon's chemical inertness.
The Significance of Neon's Full Valence Shell
Neon's electron configuration, with its completely filled outermost shell (the valence shell), is the key to its chemical behavior. The valence shell refers to the outermost shell containing electrons involved in chemical bonding. A full valence shell signifies exceptional stability. Atoms tend to react chemically to achieve a full valence shell, mimicking the stable configuration of noble gases.
Because neon already possesses a full valence shell, it has little to no tendency to gain, lose, or share electrons. This lack of reactivity makes it a noble gas, extremely unreactive under normal conditions. It's this stability that explains why neon rarely forms chemical compounds.
Neon's Properties and Applications
Neon's unique electron configuration directly impacts its various properties and consequently its applications.
Inertness and Stability:
Its chemical inertness is fundamental to many of its uses. Because it doesn't readily react with other substances, it's safe to use in various environments and applications where reactivity could be detrimental.
Gaseous State:
Neon exists as a monatomic gas under standard conditions. This is a direct consequence of its stable electron configuration—there's no energetic advantage for neon atoms to bond with each other.
Low Density:
Neon is significantly less dense than air. This property is exploited in various applications, such as in lighter-than-air devices.
Electrical Conductivity and Luminescence:
When subjected to an electric current, neon gas emits a characteristic bright reddish-orange glow. This property is utilized in neon signs, a classic and widely recognized application. The luminescence is a result of electrons being excited to higher energy levels and then releasing energy as they return to their ground state (the configuration described above).
Cryogenic Applications:
Neon's low boiling point makes it useful as a cryogenic refrigerant. Its ability to maintain extremely low temperatures finds applications in scientific research and industrial processes requiring such conditions.
Other Applications:
Neon's applications extend beyond signage and cryogenics. It's used in:
-
Helium-Neon Lasers: Neon's contribution to the He-Ne laser is crucial in producing specific wavelengths of coherent light.
-
Vacuum Tubes: Its inertness makes it suitable for use in vacuum tubes where preventing unwanted chemical reactions is essential.
Orbital Diagrams and Further Exploration
While the electron configuration (1s²2s²2p⁶) provides a concise summary, a more detailed representation involves orbital diagrams. These diagrams visually depict electron placement within individual orbitals within subshells.
For neon, the orbital diagram would show two electrons in the 1s orbital, two electrons in the 2s orbital, and six electrons distributed among the three 2p orbitals, with each orbital either empty or having one electron before pairing. This visual representation underscores Hund's rule in action.
Further exploration into neon's properties can involve investigating its spectral lines, ionization energies, and other atomic parameters that stem directly from its electronic structure. Sophisticated spectroscopic techniques allow scientists to analyze the specific energy transitions of electrons within neon atoms, reinforcing the understanding of its electron configuration.
Conclusion: Neon's Electron Configuration and its Impact
The seemingly simple electron configuration of neon, 1s²2s²2p⁶, is the foundation for understanding its unique properties and diverse applications. This completely filled valence shell is directly responsible for its exceptional inertness, stability, and distinctive behavior in electrical fields. From illuminating neon signs to cooling materials to extremely low temperatures, neon's role in modern technology and scientific research is a testament to the fundamental importance of its electron configuration. A deeper understanding of atomic structure and electron configurations provides a powerful framework for predicting and explaining the behavior of elements and their myriad applications in our world.
Latest Posts
Latest Posts
-
Confidence Interval For Two Independent Samples Calculator
Mar 28, 2025
-
Alternation Of Generations Means That Plants Produce
Mar 28, 2025
-
Feedback Loops Glucose And Glucagon Answer Key
Mar 28, 2025
-
What Are The Kinds Of Forces
Mar 28, 2025
-
Predicting Whether Molecules Are Polar Or Nonpolar
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration For Neon . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
