What Is The Electron Configuration For Sn
Muz Play
Mar 15, 2025 · 6 min read
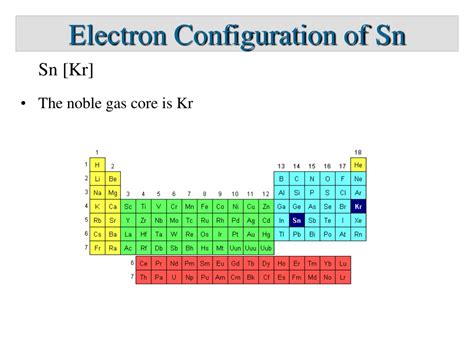
Table of Contents
What is the Electron Configuration for Sn? A Deep Dive into Tin's Atomic Structure
Tin (Sn), a fascinating element with a rich history and diverse applications, holds a unique position in the periodic table. Understanding its electron configuration is key to unlocking its chemical properties and behaviors. This article will provide a comprehensive exploration of the electron configuration of tin, delving into its underlying principles and implications. We'll explore different approaches to determining its configuration, discuss its implications for tin's reactivity, and touch upon the complexities arising from its position in the periodic table.
Understanding Electron Configuration
Before diving into tin's specific configuration, let's establish a foundational understanding of the concept. Electron configuration describes the arrangement of electrons in an atom's electron shells and subshells. It follows specific rules dictated by quantum mechanics, primarily the Aufbau principle, Hund's rule, and the Pauli exclusion principle.
-
Aufbau Principle: Electrons fill orbitals starting with the lowest energy levels. This means electrons occupy the 1s orbital before the 2s, and so on.
-
Hund's Rule: Within a subshell, electrons will individually occupy each orbital before doubling up in any one orbital. This minimizes electron-electron repulsion.
-
Pauli Exclusion Principle: No two electrons in an atom can have the same four quantum numbers (n, l, ml, and ms). This means each orbital can hold a maximum of two electrons with opposite spins.
These principles are crucial for predicting the electron configuration of any element, including tin.
Determining the Electron Configuration of Tin (Sn)
Tin has an atomic number of 50, meaning it has 50 protons and, in its neutral state, 50 electrons. To determine its electron configuration, we follow the Aufbau principle, systematically filling orbitals in order of increasing energy.
The typical representation uses a shorthand notation, indicating the electron distribution across different energy levels and subshells:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s² 4d¹⁰ 5p²
Let's break this down:
- 1s²: Two electrons in the first energy level's s subshell.
- 2s² 2p⁶: Two electrons in the second level's s subshell and six in the p subshell (a total of eight electrons filling the second shell).
- 3s² 3p⁶: Similar to the second shell, this represents the eight electrons in the third shell.
- 4s² 3d¹⁰ 4p⁶: This represents the 18 electrons in the fourth shell, including the d subshell which holds 10 electrons. Note that the 3d subshell fills after the 4s subshell due to its slightly lower energy.
- 5s² 4d¹⁰ 5p²: Finally, the fifth shell contains two electrons in the 5s subshell and two electrons in the 5p subshell. This completes the 50 electrons for a neutral tin atom.
Another way to represent the configuration is using the noble gas shorthand:
[Kr] 5s² 4d¹⁰ 5p²
Here, [Kr] represents the electron configuration of Krypton (1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶), which is a noble gas. This simplifies the notation by only specifying the electrons beyond the stable Krypton core.
Implications of Tin's Electron Configuration
Tin's electron configuration directly influences its chemical properties and reactivity. The two electrons in the 5p subshell are relatively loosely bound and are readily available for participation in chemical bonding. This explains why tin exhibits a variety of oxidation states, primarily +2 and +4.
-
+2 Oxidation State: Tin can lose its two 5p electrons, forming Sn²⁺ ions. This is common in many tin compounds.
-
+4 Oxidation State: Tin can also lose its two 5p electrons and its two 5s electrons, forming Sn⁴⁺ ions. This oxidation state is also prevalent in numerous tin compounds.
The availability of these outer electrons is the reason tin can form both ionic and covalent bonds, contributing to its versatility in chemical reactions and its ability to form a wide array of compounds.
Tin's Position in the Periodic Table and its Electron Configuration
Tin's position in the periodic table, as a post-transition metal in group 14, further illuminates its electron configuration and properties. Its placement between metals and nonmetals reflects its amphoteric nature – its ability to react with both acids and bases. The presence of the filled d subshells (4d¹⁰) contributes to its relatively lower reactivity compared to some other metals. This filled d-subshell shields the outer electrons from the nucleus, making them less tightly bound, thus affecting its chemical reactivity and contributing to its ability to exist in multiple oxidation states.
Variations and Exceptions
While the standard electron configuration presented above is generally accepted, it's important to note that the energy levels of orbitals can vary slightly depending on the specific chemical environment of the tin atom. In certain compounds or complex ions, slight deviations from the standard configuration might be observed due to ligand field effects and other interatomic interactions. However, these variations are generally minor and do not fundamentally alter the overall understanding of tin's electronic structure and chemical behavior.
Applications of Tin and its Relationship to Electron Configuration
Understanding the electron configuration of tin helps us comprehend its wide range of applications. These include:
-
Solder: Tin's ability to form alloys with low melting points makes it crucial in soldering applications, connecting electronic components and other metallic parts. This property relates directly to its ability to form metallic bonds.
-
Coatings: Tin coatings protect other metals from corrosion, taking advantage of its oxidation properties and resistance to environmental factors.
-
Food Packaging: Tinplate (tin-coated steel) is widely used in food cans because of tin's non-toxicity and its barrier properties, preventing food spoilage.
-
Organotin Compounds: These compounds find use as biocides (pesticides, fungicides) and catalysts, making use of tin's bonding versatility stemming from its multiple oxidation states. The reactivity associated with specific oxidation states is crucial in designing effective organotin compounds.
Conclusion: The Significance of Tin's Electron Configuration
The electron configuration of tin ([Kr] 5s² 4d¹⁰ 5p²) is far more than just a numerical representation; it is a key to understanding its chemical behavior, reactivity, and diverse applications. By systematically filling orbitals according to the principles of quantum mechanics, we can predict its oxidation states, bonding capabilities, and ultimately, its role in various industrial and technological processes. The unique properties derived from its electronic structure cement its importance in numerous fields, showcasing the fundamental connection between atomic structure and macroscopic properties. This deep understanding, gained through comprehending its electron configuration, allows us to harness tin’s unique characteristics for a variety of beneficial purposes. From everyday applications like food cans to advanced technology in electronics, the role of tin is intimately linked to the arrangement of its electrons.
Latest Posts
Latest Posts
-
How To Calculate The Density Of A Rock
Mar 15, 2025
-
Recall That In Cellular Respiration The Processes Of Glycolysis
Mar 15, 2025
-
How Many Unpaired Electrons Are In Sulfur Atom
Mar 15, 2025
-
Similarities Between The Romanticism And Transcendentalism Movement
Mar 15, 2025
-
Made Up Of Two Glucose Polysaccharides Amylose And Amylopectin
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration For Sn . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
