What Is The Kirby Bauer Test
Muz Play
Mar 21, 2025 · 7 min read
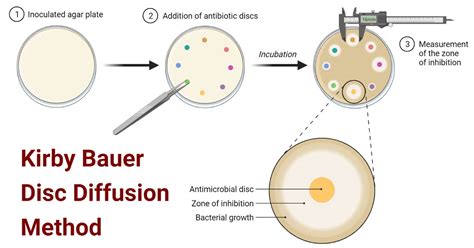
Table of Contents
What is the Kirby-Bauer Test? Your Comprehensive Guide
The Kirby-Bauer test, also known as the disk diffusion test, is a widely used method in microbiology laboratories to determine the susceptibility of bacteria to various antimicrobial agents. This crucial test helps clinicians select the most effective antibiotic to treat bacterial infections, guiding treatment decisions and contributing significantly to patient care. Understanding the principles, procedure, and interpretation of the Kirby-Bauer test is vital for anyone working in the field of infectious diseases or microbiology.
Understanding the Principles Behind the Kirby-Bauer Test
The Kirby-Bauer test relies on the principle of diffusion. Antimicrobial disks containing a known concentration of an antibiotic are placed onto an agar plate that has been evenly inoculated with the bacterial isolate being tested. As the antibiotic diffuses outward from the disk, it creates a concentration gradient. If the bacteria are susceptible to the antibiotic, a zone of inhibition—a clear area around the disk where bacterial growth is inhibited—will appear. The size of this zone of inhibition is directly related to the susceptibility of the bacteria to the antibiotic. Larger zones indicate greater susceptibility.
Key Factors Influencing Zone of Inhibition Size
Several factors influence the size of the zone of inhibition, including:
- Antibiotic concentration: Higher concentrations of the antibiotic will generally result in larger zones of inhibition.
- Diffusibility of the antibiotic: Some antibiotics diffuse more readily through agar than others.
- Bacterial growth rate: Rapidly growing bacteria may exhibit smaller zones of inhibition.
- Inoculum size: A denser inoculum will result in smaller zones of inhibition.
- Agar depth: The depth of the agar influences the diffusion of the antibiotic. Too thick or too thin an agar layer can lead to inaccurate results.
- Incubation temperature and time: Inconsistent incubation conditions can affect the bacterial growth and the size of the inhibition zone.
The Step-by-Step Kirby-Bauer Test Procedure
The Kirby-Bauer test requires meticulous execution to ensure accurate and reliable results. Here’s a detailed breakdown of the procedure:
1. Preparing the Bacterial Inoculum
The first step involves preparing a standardized bacterial suspension. This is crucial because the inoculum density directly impacts the zone of inhibition. A turbidity standard, often using a 0.5 McFarland standard, is used to achieve the correct bacterial concentration. This ensures consistent and comparable results across different tests. This standardized suspension is then swabbed evenly onto the Mueller-Hinton agar plate.
2. Selecting and Placing the Antibiotic Disks
Pre-prepared antibiotic disks containing specific concentrations of antimicrobial agents are used. The choice of antibiotics will depend on the suspected bacterial pathogen and the clinical context. The disks are carefully placed onto the inoculated agar plate using sterile forceps. A standardized distance must be maintained between the disks to avoid overlapping zones of inhibition. The disks should be pressed gently to ensure proper contact with the agar surface.
3. Incubation
The inoculated agar plate is then incubated under controlled conditions—usually at 35°C for 16-18 hours. This allows sufficient time for bacterial growth and antibiotic diffusion. Consistency in incubation conditions is paramount for accurate results.
4. Measuring and Interpreting Zones of Inhibition
After the incubation period, the zones of inhibition are carefully measured in millimeters using a ruler. The diameter of each zone is recorded, not the radius. The size of the zones is then compared to standardized interpretive charts provided by the Clinical and Laboratory Standards Institute (CLSI). These charts define the zones of inhibition that correlate to susceptible (S), intermediate (I), and resistant (R) categories for each antibiotic-bacteria combination.
Interpreting the Results: Susceptible, Intermediate, or Resistant
The interpretation of the Kirby-Bauer test results is based on the diameter of the zone of inhibition and the CLSI interpretive charts.
-
Susceptible (S): A large zone of inhibition indicates that the bacteria are susceptible to the antibiotic. The antibiotic is likely to be effective in treating the infection.
-
Intermediate (I): A moderate zone of inhibition suggests intermediate susceptibility. The antibiotic might be effective in certain clinical situations, such as high-concentration therapy or local infection sites, but might not be the optimal choice.
-
Resistant (R): A small or no zone of inhibition indicates that the bacteria are resistant to the antibiotic. The antibiotic is unlikely to be effective in treating the infection. Alternative antibiotics should be considered.
It's crucial to remember that the Kirby-Bauer test results should be interpreted in conjunction with other clinical information, such as the patient's history, symptoms, and other laboratory findings. The test provides valuable information about the antimicrobial susceptibility of the bacteria, but it is not the sole factor in determining the optimal treatment strategy.
Importance of Quality Control in Kirby-Bauer Testing
Maintaining quality control is essential to ensure the accuracy and reliability of Kirby-Bauer tests. This involves using standardized methods, reagents, and equipment. Quality control measures include:
- Using properly prepared Mueller-Hinton agar: The agar should meet specific requirements regarding depth and composition.
- Employing standardized inoculum preparation: Consistent bacterial concentration is critical.
- Using certified antibiotic disks: Disks from reputable manufacturers should be used to ensure accurate antibiotic concentrations.
- Regularly testing quality control strains: Known susceptible and resistant bacterial strains are tested to verify the accuracy of the test procedure and reagents. This helps to detect any potential problems with the methodology or materials.
Limitations of the Kirby-Bauer Test
While the Kirby-Bauer test is a valuable tool, it has limitations:
- It doesn't account for pharmacokinetic and pharmacodynamic factors: The test doesn't reflect how the antibiotic behaves in the body, such as its distribution, metabolism, and excretion.
- It may not accurately predict the clinical outcome: While the test provides information on in vitro susceptibility, the actual clinical response can vary.
- It doesn't detect all mechanisms of resistance: Some bacterial resistance mechanisms might not be fully detected by this method.
- It is limited to bacteria that grow on agar plates: The test is not suitable for fastidious bacteria or those that require specialized culture conditions.
The Kirby-Bauer Test and Antibiotic Stewardship
The judicious use of antibiotics is crucial to combat the growing threat of antibiotic resistance. The Kirby-Bauer test plays a significant role in antibiotic stewardship by providing information that guides clinicians in making informed decisions about antibiotic selection and usage. By selecting the most appropriate antibiotic based on the susceptibility profile of the infecting bacterium, clinicians can optimize treatment efficacy, minimize the development of antibiotic resistance, and improve patient outcomes. Inappropriate antibiotic use contributes to the rise of antibiotic-resistant bacteria, which pose a major public health challenge.
Advances and Future Directions in Antibiotic Susceptibility Testing
The Kirby-Bauer test remains a cornerstone of antibiotic susceptibility testing, but advancements are continually being made. These include:
- Automated systems: Automated systems are being developed to increase throughput and reduce the risk of human error.
- Molecular techniques: Molecular methods are being used to identify specific resistance genes, providing a more detailed understanding of the resistance mechanisms.
- New technologies: Novel approaches such as microfluidic devices and high-throughput screening methods are being investigated to improve the efficiency and accuracy of antibiotic susceptibility testing.
These advances will enhance the speed, accuracy, and information content of antibiotic susceptibility testing, contributing to better infection control and improved patient care.
Conclusion
The Kirby-Bauer test is an essential tool in microbiology laboratories worldwide. Its simplicity, affordability, and reliability make it invaluable for determining the susceptibility of bacteria to various antimicrobial agents. While it has limitations, the results, when interpreted correctly in conjunction with other clinical information, significantly impact treatment decisions and contribute to effective antibiotic stewardship. Ongoing advancements in technology continue to refine and expand the capabilities of this fundamental test, ensuring its continued relevance in combating bacterial infections in the future. The accurate and timely performance of this test remains critical for successful patient management and the global fight against antimicrobial resistance.
Latest Posts
Latest Posts
-
How Does Thermal Energy Affect The 3 States Of Matter
Mar 21, 2025
-
Is Solubility A Chemical Or Physical Property
Mar 21, 2025
-
What Are Vertical Columns On The Periodic Table Called
Mar 21, 2025
-
What Is The Stage On A Microscope
Mar 21, 2025
-
Dimensional Analysis Problems With Answers Pdf
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about What Is The Kirby Bauer Test . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
