What Are Vertical Columns On The Periodic Table Called
Muz Play
Mar 21, 2025 · 6 min read
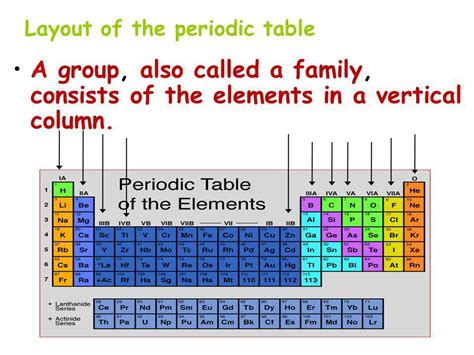
Table of Contents
What Are Vertical Columns on the Periodic Table Called? A Deep Dive into Groups and Families
The periodic table, that iconic grid of chemical elements, is more than just a neatly organized list. It's a powerful tool that reveals fundamental relationships between atoms, predicting their properties and behaviors. Understanding its structure is key to grasping the fundamentals of chemistry. A crucial aspect of this structure is the organization into vertical columns, which are not just randomly placed but represent elements with shared characteristics. So, what are these vertical columns called? They are known as groups, or sometimes families. Let's delve deeper into their significance and the nuances within this terminology.
Groups: A Shared Electron Configuration Story
The elements within a group share a similar electron configuration in their outermost shell, also known as the valence shell. This shared electron configuration is the driving force behind their strikingly similar chemical properties. The number of electrons in the valence shell dictates how an atom will interact with other atoms – will it readily lose electrons, gain electrons, or share them? This determines an element's reactivity, its tendency to form chemical bonds, and the types of compounds it will form.
Why Electron Configuration Matters
The electrons in the valence shell are the ones involved in chemical bonding. Elements in the same group have the same number of valence electrons. For example, all elements in Group 1 (alkali metals) have one valence electron, leading to their high reactivity and tendency to lose that single electron to form +1 ions. Similarly, Group 18 (noble gases) have a full valence shell, making them exceptionally stable and unreactive.
Predicting Properties Based on Group Membership
Knowing the group an element belongs to allows chemists to make educated predictions about its properties. For instance, if we know an element belongs to Group 17 (halogens), we can anticipate that it will be highly reactive, a strong oxidizing agent, and likely to form -1 ions. This predictive power is a cornerstone of the periodic table's utility in chemical research and understanding. This ability to predict behavior based on group membership saves significant time and resources in experimental research.
Families: Another Name, Same Meaning
While "group" is the most commonly used and accepted term, you might also encounter the term "family" to describe these vertical columns. Essentially, "family" is a more informal and less precise term conveying the same fundamental concept. Both terms highlight the shared chemical characteristics and behaviors of the elements within the column.
The Nuances in Terminology
While interchangeable in many contexts, the use of "family" can sometimes lead to ambiguity. The term "family" might evoke a sense of looser relationships than the more rigorously defined "group," which emphasizes the specific electron configuration similarity. Therefore, in formal scientific writing, "group" is preferred for its precision. However, in educational settings or introductory discussions, "family" might be used to make the concept more approachable.
A Deeper Look at Specific Groups
Let's explore some key groups and families to illustrate the power of this organizational system:
Group 1: The Alkali Metals
The alkali metals (lithium, sodium, potassium, rubidium, cesium, and francium) are all highly reactive metals. Their single valence electron makes them eager to lose it and form +1 ions. This reactivity increases as you go down the group. These elements are soft, silvery-white metals that react vigorously with water, producing hydrogen gas.
Group 2: The Alkaline Earth Metals
Slightly less reactive than the alkali metals, the alkaline earth metals (beryllium, magnesium, calcium, strontium, barium, and radium) possess two valence electrons. They tend to lose these two electrons to form +2 ions. These metals are also silvery-white but generally harder and denser than the alkali metals.
Group 17: The Halogens
The halogens (fluorine, chlorine, bromine, iodine, and astatine) are highly reactive nonmetals. They have seven valence electrons, and their strong tendency to gain one electron to achieve a full octet makes them powerful oxidizing agents. Their reactivity decreases down the group. Halogens exist in various states of matter at room temperature – fluorine and chlorine are gases, bromine is a liquid, and iodine is a solid.
Group 18: The Noble Gases
The noble gases (helium, neon, argon, krypton, xenon, and radon) are unique in their exceptional inertness. They possess a full valence shell, making them extremely stable and unreactive. This full octet explains their lack of participation in typical chemical bonding. Historically considered completely inert, some heavier noble gases have been shown to form compounds under specific conditions.
The Importance of Group Trends
Understanding the trends within groups is crucial for predicting chemical behavior. Several key trends exist:
- Atomic Radius: Generally, atomic radius increases as you go down a group. This is because each successive element adds an electron shell.
- Electronegativity: Electronegativity, the ability of an atom to attract electrons in a chemical bond, generally decreases as you go down a group.
- Ionization Energy: The energy required to remove an electron from an atom decreases as you go down a group.
- Melting and Boiling Points: These properties show varying trends depending on the specific group.
Beyond Groups: Understanding Periods
While groups are crucial, it's essential to remember that the periodic table is organized both vertically (groups/families) and horizontally (periods). Periods represent elements with the same number of electron shells. Elements within a period exhibit trends in their properties as you move across from left to right, reflecting the gradual filling of the electron shells. Understanding both the group and period relationships is essential to mastering the predictive power of the periodic table.
The Periodic Table: A Dynamic Tool
The periodic table is a constantly evolving instrument reflecting our ongoing understanding of chemistry and the elements. New elements are discovered, and our comprehension of existing elements deepens. However, the fundamental organization into groups and families remains a cornerstone of its power. Understanding that the vertical columns are called groups (or families) is merely the first step in unlocking the vast insights offered by this incredible organizational tool.
Conclusion: Groups and Families – The Cornerstones of Chemical Understanding
In summary, the vertical columns of the periodic table are known as groups, or sometimes families. This organization is fundamental to understanding the relationships between elements and predicting their properties based on their shared electron configurations. The similarity in valence electron numbers within a group accounts for the similar chemical and physical properties observed across the elements within the same column. While “family” is a less formal, more colloquial term, “group” remains the preferred and scientifically precise term when discussing this aspect of the periodic table's structure. The periodic table, with its systematic organization into groups and periods, serves as a powerful testament to the elegance and order underlying the complexity of chemical interactions. Continued exploration and understanding of these relationships will remain crucial to advancements in chemistry and related fields.
Latest Posts
Latest Posts
-
The Basic Functional Unit Of The Kidney Is The
Mar 22, 2025
-
Bohr Diagrams For The First 20 Elements
Mar 22, 2025
-
What Is The Property Of Bases
Mar 22, 2025
-
Is A Cow A First Level Consumer
Mar 22, 2025
-
What Is The Heat Of Vaporization Of Water
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about What Are Vertical Columns On The Periodic Table Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
