What Is The Mass Percent Of Chlorine In Hydrochloric Acid
Muz Play
Mar 17, 2025 · 5 min read
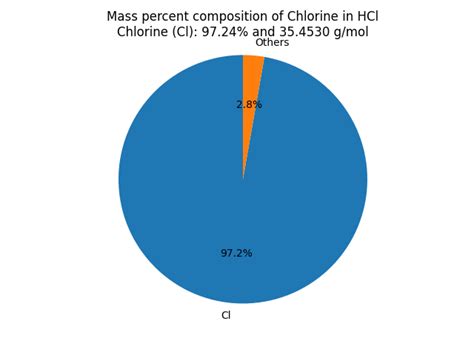
Table of Contents
What is the Mass Percent of Chlorine in Hydrochloric Acid?
Hydrochloric acid (HCl), a strong and highly corrosive mineral acid, finds extensive applications in various industrial processes and laboratories. Understanding its composition, particularly the mass percent of chlorine, is crucial for accurate calculations and safe handling. This article delves deep into calculating and understanding the mass percent of chlorine in hydrochloric acid, exploring the concept, the calculation method, and its implications.
Understanding Mass Percent
Before diving into the specifics of hydrochloric acid, let's establish a clear understanding of mass percent. Mass percent, also known as weight percent, represents the mass of a particular component in a mixture or compound expressed as a percentage of the total mass. The formula for calculating mass percent is:
(Mass of component / Total mass of mixture) x 100%
This simple yet powerful formula is the foundation for determining the chlorine content in hydrochloric acid.
Hydrochloric Acid: A Closer Look
Hydrochloric acid is an aqueous solution of hydrogen chloride (HCl) gas. Pure hydrogen chloride is a colorless gas, but when dissolved in water, it forms hydrochloric acid. The concentration of hydrochloric acid is typically expressed as molarity (moles per liter) or percentage by weight (mass percent). The mass percent signifies the percentage of HCl by mass in the total solution.
Important Note: It is crucial to differentiate between the mass percent of HCl and the mass percent of chlorine. While both are related, they are not the same. The mass percent of HCl represents the percentage of hydrogen chloride in the solution, whereas the mass percent of chlorine represents the percentage of chlorine atoms within the HCl molecules.
Calculating the Mass Percent of Chlorine in Hydrochloric Acid
To calculate the mass percent of chlorine in hydrochloric acid, we need to consider the molar mass of chlorine (Cl) and the molar mass of hydrogen chloride (HCl).
- Molar mass of Chlorine (Cl): Approximately 35.45 g/mol
- Molar mass of Hydrogen (H): Approximately 1.01 g/mol
- Molar mass of Hydrogen Chloride (HCl): Approximately 36.46 g/mol (1.01 g/mol + 35.45 g/mol)
The mass percent of chlorine in pure HCl can be calculated as follows:
(Molar mass of Cl / Molar mass of HCl) x 100%
= (35.45 g/mol / 36.46 g/mol) x 100%
≈ 97.2%
This means that in pure hydrogen chloride, approximately 97.2% of its mass is contributed by chlorine.
However, commercially available hydrochloric acid is not pure HCl. It's an aqueous solution, meaning it's dissolved in water. The concentration of commercially available hydrochloric acid is typically expressed as a percentage by weight (e.g., 37% HCl). This signifies that 37 grams of HCl are present in 100 grams of the solution. The remaining 63 grams are water.
Therefore, to determine the mass percent of chlorine in a specific concentration of hydrochloric acid (e.g., 37% HCl), we need a two-step calculation:
Step 1: Determine the mass of HCl in the solution.
For a 37% HCl solution:
- Assume a 100g sample of the solution.
- Mass of HCl = 37% of 100g = 37g
Step 2: Determine the mass of chlorine in the HCl.
- Using the molar mass ratio from earlier, we know that in 36.46g of HCl, there are 35.45g of chlorine.
- Using proportions: (35.45g Cl / 36.46g HCl) = (x g Cl / 37g HCl)
- Solving for x: x = (35.45g Cl * 37g HCl) / 36.46g HCl ≈ 36.0g Cl
Step 3: Calculate the mass percent of chlorine in the 37% HCl solution.
- Mass percent of chlorine = (mass of chlorine / total mass of solution) x 100%
- Mass percent of chlorine = (36.0g Cl / 100g solution) x 100% = 36.0%
Therefore, a 37% HCl solution contains approximately 36.0% chlorine by mass. Remember that this calculation assumes the solution contains only HCl and water. Impurities will affect this calculation.
Factors Affecting the Mass Percent of Chlorine
Several factors can influence the precise mass percent of chlorine in a hydrochloric acid solution:
-
Concentration of HCl: As demonstrated, the concentration of HCl directly impacts the mass percent of chlorine. A higher concentration of HCl leads to a higher mass percent of chlorine.
-
Purity of HCl: The presence of impurities in the hydrochloric acid solution can alter the mass percent of chlorine. Impurities can dilute the concentration of HCl, reducing the overall chlorine content.
-
Temperature: Temperature affects the density of the solution, which in turn can slightly alter the mass percent calculation. However, this effect is usually negligible for most practical purposes.
-
Manufacturing Process: Variations in the manufacturing process can lead to slight differences in the concentration and purity of the hydrochloric acid produced, affecting the final mass percent of chlorine.
Importance of Knowing the Mass Percent of Chlorine
Accurately knowing the mass percent of chlorine in hydrochloric acid is essential in various contexts:
-
Stoichiometric Calculations: In chemical reactions involving hydrochloric acid, the mass percent of chlorine is crucial for accurate stoichiometric calculations to determine reactant and product amounts.
-
Industrial Applications: Industries using hydrochloric acid in large quantities (e.g., metal processing, food processing, and pharmaceuticals) need precise information about the chlorine content for process optimization, quality control, and safety regulations.
-
Safety and Handling: The corrosive nature of hydrochloric acid necessitates careful handling. Understanding the chlorine content helps in assessing the potential hazards and implementing appropriate safety measures.
-
Environmental Regulations: Accurate determination of chlorine content is vital for environmental compliance as it's essential for waste management and environmental impact assessments.
Conclusion
Determining the mass percent of chlorine in hydrochloric acid involves a straightforward calculation based on the molar masses of chlorine and hydrogen chloride. However, it's crucial to remember that commercially available hydrochloric acid is an aqueous solution, and its concentration needs to be factored into the calculation. The mass percent of chlorine is a critical parameter with significant implications for various applications, emphasizing the importance of accurate measurement and understanding of this fundamental chemical property. Understanding the factors that influence this percentage ensures safe and efficient usage of this highly important chemical. This information empowers scientists, engineers, and technicians to work safely and effectively with this powerful chemical.
Latest Posts
Latest Posts
-
How To Compute Cost Per Equivalent Unit
Mar 18, 2025
-
What Are The Physical Properties Of A Metal
Mar 18, 2025
-
Why Is The Force Subscript Not Written In The Us
Mar 18, 2025
-
A Relationship In Which Both Organisms Benefit
Mar 18, 2025
-
If One Of The Reactants In A Reaction Is
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about What Is The Mass Percent Of Chlorine In Hydrochloric Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
