What Is The Number Of Protons In Beryllium
Muz Play
Mar 30, 2025 · 5 min read
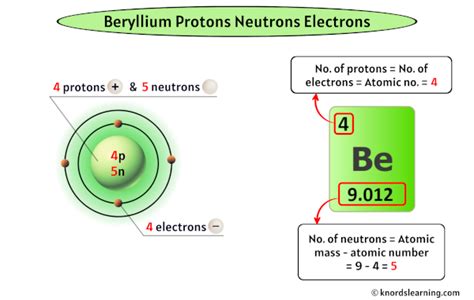
Table of Contents
What is the Number of Protons in Beryllium? A Deep Dive into Atomic Structure
Beryllium, a fascinating element with unique properties, holds a significant place in various fields, from aerospace engineering to nuclear technology. Understanding its atomic structure is fundamental to appreciating its behavior and applications. This article will delve deep into the question: What is the number of protons in beryllium? We'll explore not just the answer but also the broader implications of this fundamental characteristic, connecting it to beryllium's position in the periodic table, its isotopes, and its overall chemical behavior.
Understanding Atomic Number and Protons
At the heart of any atom lies its nucleus, a dense core containing two types of subatomic particles: protons and neutrons. The number of protons in an atom's nucleus defines its atomic number, a fundamental property that uniquely identifies each element. This number is so crucial because it dictates the element's chemical properties and its placement on the periodic table. The periodic table itself is organized based on increasing atomic number.
Therefore, to answer the question, "What is the number of protons in beryllium?", we need to find beryllium's atomic number.
Beryllium's Position on the Periodic Table and its Atomic Number
Beryllium (Be) is located in the second position on the periodic table. This placement directly reveals its atomic number: 4. This means that a beryllium atom contains 4 protons. This fundamental fact underpins all the other properties and behaviors of beryllium.
The Significance of Atomic Number 4
The atomic number 4 is not arbitrary. It dictates the number of electrons in a neutral beryllium atom (also 4), which in turn determines its electronic configuration and chemical reactivity. This configuration influences how beryllium forms chemical bonds, its valency (the number of bonds it can form), and its overall chemical behavior.
Isotopes of Beryllium: A Deeper Look into Nuclear Structure
While the number of protons defines the element, the number of neutrons can vary. Atoms of the same element with different numbers of neutrons are called isotopes. Beryllium has several known isotopes, all sharing the same number of protons (4), but differing in their neutron count.
Common Isotopes of Beryllium:
-
Beryllium-9 (⁹Be): This is the most stable and abundant isotope of beryllium, accounting for almost 100% of naturally occurring beryllium. It has 4 protons and 5 neutrons.
-
Beryllium-10 (¹⁰Be): This is a radioactive isotope of beryllium, formed in the upper atmosphere through cosmic ray spallation. It has 4 protons and 6 neutrons. Its long half-life (1.39 million years) makes it useful in various dating techniques.
The existence of isotopes doesn't change the fundamental answer to our question: the number of protons in beryllium remains 4, regardless of the specific isotope.
Beryllium's Electronic Configuration and Chemical Behavior
Beryllium's atomic number of 4 directly influences its electronic configuration. Its electrons are arranged in two energy levels (shells): two electrons in the first shell (1s²) and two electrons in the second shell (2s²). This configuration explains beryllium's characteristic chemical behavior.
Chemical Reactivity and Bonding
Beryllium's electronic configuration indicates that it has two valence electrons in its outermost shell. This means it readily forms covalent bonds to achieve a stable octet (a full outer shell) by sharing its two electrons. However, beryllium's small atomic size and high ionization energy make it relatively unreactive compared to other alkaline earth metals. It readily forms compounds with highly electronegative elements like oxygen and fluorine, displaying a +2 oxidation state.
Applications Leveraging Beryllium's Properties
The unique properties of beryllium, directly stemming from its atomic structure, make it invaluable in various applications:
-
Aerospace: Its high strength-to-weight ratio and stiffness make it ideal for lightweight aerospace components.
-
Nuclear Technology: Its low neutron absorption cross-section is crucial in nuclear reactors and neutron moderators.
-
Electronics: Its high thermal conductivity is utilized in heat sinks and other electronic applications.
-
Medical Imaging: Some beryllium compounds have been used in X-ray applications, although precautions are necessary due to its toxicity.
These applications highlight the importance of understanding beryllium's atomic structure and properties. The fundamental number of protons (4) is the key that unlocks its unique behavior and its wide-ranging applications.
Beryllium's Toxicity and Safety Precautions
While beryllium offers significant advantages in various technological fields, it's essential to acknowledge its potential health hazards. Beryllium and its compounds are toxic, and exposure can lead to chronic beryllium disease (CBD), a serious lung condition. Proper safety measures and handling procedures are crucial when working with beryllium.
Understanding and Mitigating Beryllium Toxicity
The toxicity of beryllium stems from its interaction with biological systems at the cellular level. Strict regulations and guidelines are in place to control exposure and protect workers from its hazardous effects. Proper ventilation, personal protective equipment (PPE), and careful waste management are vital aspects of safe beryllium handling.
Conclusion: The Significance of Four Protons
In summary, the number of protons in beryllium is definitively 4. This seemingly simple number is the cornerstone of beryllium's identity, shaping its position on the periodic table, its isotopic variations, its electronic configuration, and ultimately, its chemical and physical properties. Understanding this fundamental characteristic is essential for comprehending beryllium's role in various scientific and technological advancements, as well as for ensuring safe handling and mitigation of its potential health risks. The number 4 is not just a numerical value; it is the key to unlocking the unique potential and challenges presented by this remarkable element. From aerospace components to nuclear reactors, the influence of those four protons is undeniable. This detailed exploration of beryllium's atomic structure emphasizes the importance of fundamental scientific concepts in understanding the macroscopic properties and applications of the elements around us.
Latest Posts
Latest Posts
-
Elements Are Organized On The Periodic Table According To
Apr 01, 2025
-
The Envelope Of A Virus Is Derived From The Hosts
Apr 01, 2025
-
Plant Cell In A Hypotonic Solution
Apr 01, 2025
-
What Is The Definition Of Form In Music
Apr 01, 2025
-
Staph Epidermidis Hemolysis On Blood Agar
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Is The Number Of Protons In Beryllium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
