Elements Are Organized On The Periodic Table According To
Muz Play
Apr 01, 2025 · 6 min read
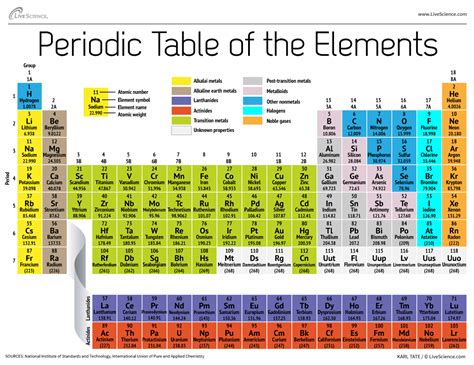
Table of Contents
Elements are Organized on the Periodic Table According to: A Deep Dive into the Organization of the Periodic Table
The periodic table, a cornerstone of chemistry, isn't just a random arrangement of elements. Its structured organization reflects fundamental properties and behaviors of atoms, providing a powerful tool for understanding chemical reactions and predicting element properties. This comprehensive guide delves deep into the principles underlying the periodic table's organization, exploring the factors that dictate the placement of each element and the insightful information it reveals.
The Genesis of the Periodic Table: Mendeleev's Vision
Before delving into the intricacies of modern organization, it's essential to acknowledge the pioneering work of Dmitri Mendeleev. In the late 19th century, the number of known elements was growing rapidly, leading to confusion and a lack of systematic understanding. Mendeleev, recognizing patterns in the elements' properties, arranged them in order of increasing atomic weight, noting recurring trends. This resulted in his groundbreaking periodic table, where elements with similar properties appeared in vertical columns, or groups.
Mendeleev's brilliance wasn't just in his arrangement; he also predicted the existence and properties of elements yet to be discovered, leaving gaps in his table with placeholders for these "missing" elements. These predictions were later confirmed, solidifying the validity and predictive power of his periodic system.
The Modern Periodic Table: Atomic Number Takes Center Stage
While Mendeleev's table was revolutionary, the modern periodic table is organized primarily by atomic number, not atomic weight. The atomic number represents the number of protons in an atom's nucleus, a fundamental characteristic that uniquely identifies each element. This crucial shift clarified certain inconsistencies in Mendeleev's original arrangement, stemming from the fact that atomic weight doesn't always perfectly reflect the order of increasing proton number.
Atomic Structure and Periodic Trends: The Foundation of Organization
The periodic table's organization hinges on the arrangement of electrons within an atom. Electrons occupy specific energy levels, or shells, surrounding the nucleus. Each shell can accommodate a specific number of electrons, and the outermost shell, the valence shell, plays a crucial role in determining an element's chemical behavior.
The number of valence electrons dictates an element's group or column in the periodic table. Elements within the same group possess similar valence electron configurations, leading to similar chemical properties. For instance, elements in Group 1 (alkali metals) all have one valence electron, leading to their high reactivity.
Key Organizational Principles of the Periodic Table
The organization of the periodic table isn't simply linear; it's a multi-faceted system incorporating several fundamental principles:
1. Increasing Atomic Number: The Primary Ordering Principle
As previously mentioned, the primary organizational principle is the increasing atomic number. This ensures that elements are arranged in a logical sequence, reflecting the fundamental building blocks of matter. The sequential arrangement is critical for understanding the periodic trends in properties.
2. Periods: Horizontal Rows Reflecting Energy Levels
The horizontal rows, or periods, represent the principal energy levels occupied by electrons. Each period begins with an element whose valence electrons start filling a new shell. As you move across a period, the number of protons and electrons increases, leading to changes in atomic radius, electronegativity, and ionization energy. The length of each period correlates with the maximum number of electrons that can occupy the electron shells.
3. Groups: Vertical Columns of Shared Chemical Behavior
The vertical columns, or groups, are where elements with similar chemical properties reside. This similarity stems from their identical number of valence electrons. Elements within the same group tend to exhibit similar reactivity and form similar types of compounds.
- Group 1 (Alkali Metals): Highly reactive metals with one valence electron.
- Group 2 (Alkaline Earth Metals): Reactive metals with two valence electrons.
- Group 17 (Halogens): Highly reactive nonmetals with seven valence electrons.
- Group 18 (Noble Gases): Inert gases with full valence shells, making them exceptionally unreactive.
4. Blocks: Subshells and Electron Configurations
The periodic table is also divided into blocks, representing the subshells within each energy level where the valence electrons reside. These blocks are designated as s, p, d, and f blocks.
- s-block: Includes Groups 1 and 2, characterized by filling the s subshell.
- p-block: Includes Groups 13-18, characterized by filling the p subshell.
- d-block: Includes the transition metals, characterized by filling the d subshell.
- f-block: Includes the lanthanides and actinides (rare earth elements), characterized by filling the f subshell.
5. Metals, Nonmetals, and Metalloids: A Classification Based on Properties
The periodic table also visually separates elements into metals, nonmetals, and metalloids based on their physical and chemical properties.
- Metals: Typically located on the left side of the table, metals are characterized by their luster, conductivity, malleability, and ductility. They tend to lose electrons readily, forming positive ions (cations).
- Nonmetals: Located primarily on the right side of the table, nonmetals lack the metallic properties of metals. They often gain electrons to form negative ions (anions).
- Metalloids (Semimetals): Found along the "stair-step" line separating metals and nonmetals, metalloids exhibit properties intermediate between metals and nonmetals. Their properties can vary depending on conditions, making them useful in semiconductors and other technological applications.
Periodic Trends: Unveiling Patterns in Element Properties
The organization of the periodic table allows us to observe and predict periodic trends in element properties. These trends are systematic changes in properties as you move across a period or down a group. Understanding these trends is crucial for predicting chemical behavior.
1. Atomic Radius: Size Matters
Atomic radius refers to the size of an atom. As you move down a group, the atomic radius generally increases due to the addition of new electron shells. However, as you move across a period, the atomic radius generally decreases due to the increasing nuclear charge pulling the electrons closer to the nucleus.
2. Ionization Energy: The Energy Cost of Removing Electrons
Ionization energy is the energy required to remove an electron from an atom. Generally, ionization energy increases as you move across a period (due to increasing nuclear charge) and decreases as you move down a group (due to increasing atomic radius and shielding effect).
3. Electronegativity: Electron-Grabbing Power
Electronegativity measures an atom's ability to attract electrons in a chemical bond. Electronegativity generally increases as you move across a period (due to increasing nuclear charge) and decreases as you move down a group (due to increasing atomic radius).
4. Electron Affinity: Attraction for Added Electrons
Electron affinity is the energy change associated with adding an electron to an atom. It generally increases as you move across a period and decreases as you move down a group, following similar trends to electronegativity.
The Periodic Table: A Dynamic and Evolving Resource
The periodic table is not a static entity; it continues to evolve with new discoveries and a deeper understanding of atomic structure and chemical behavior. The synthesis of new elements, particularly the superheavy elements, continues to push the boundaries of the table, offering exciting opportunities for scientific exploration.
The periodic table remains an invaluable tool for chemists, physicists, and other scientists, enabling them to predict and understand the behavior of matter. Its organization, based on atomic number, electron configuration, and periodic trends, allows for a systematic and insightful approach to the study of elements and their interactions, underpinning countless advancements in science and technology. Further research and exploration promise to reveal even deeper insights into the fundamental nature of matter, enhancing our understanding of the universe and its components. The periodic table, in its elegant simplicity, continues to serve as a testament to the power of scientific organization and prediction.
Latest Posts
Latest Posts
-
What Is A Non Rigid Transformation
Apr 02, 2025
-
Write The Equation In Spherical Coordinates
Apr 02, 2025
-
In What Form Do Fats First Enter The Bloodstream
Apr 02, 2025
-
Where Are The Alkaline Earth Metals On The Periodic Table
Apr 02, 2025
-
Reacts With Acid Physical Or Chemical
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Elements Are Organized On The Periodic Table According To . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
