What Is The Number Of Protons In Oxygen
Muz Play
Mar 16, 2025 · 5 min read
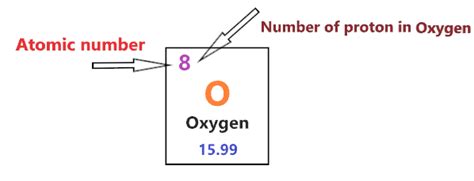
Table of Contents
What is the Number of Protons in Oxygen? A Deep Dive into Atomic Structure
Oxygen, the life-giving element crucial for respiration in most living organisms, holds a fundamental place in chemistry and biology. Understanding its atomic structure, particularly the number of protons it possesses, is key to grasping its properties and behavior. This article will delve deep into the question: What is the number of protons in oxygen? We’ll explore not just the answer but also the broader context of atomic numbers, isotopes, and the implications of proton count on oxygen's characteristics.
Understanding Atomic Structure: The Foundation of Oxygen's Identity
Before we pinpoint the number of protons in oxygen, it's crucial to establish a firm understanding of atomic structure. An atom, the fundamental unit of matter, comprises three subatomic particles:
- Protons: Positively charged particles residing in the atom's nucleus. The number of protons defines the element itself.
- Neutrons: Neutral particles also located in the nucleus. They contribute to the atom's mass but not its charge.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels. They determine an atom's chemical reactivity.
The atomic number of an element is the number of protons found in the nucleus of a single atom of that element. This number is unique to each element and is fundamental to its identity. It's like a fingerprint for an element, distinguishing it from all others. Elements are organized on the periodic table according to their atomic numbers, increasing from left to right and top to bottom.
The Answer: The Number of Protons in Oxygen
The atomic number of oxygen is 8. This unequivocally means that a single atom of oxygen contains 8 protons. This fundamental characteristic is what differentiates oxygen from all other elements. Whether it's in the air we breathe, the water we drink, or the countless organic molecules that make up life, oxygen's eight protons define its chemical behavior and place in the universe.
Isotopes: Variations on a Theme
While the number of protons remains constant for a given element, the number of neutrons can vary. Atoms of the same element with differing numbers of neutrons are called isotopes. Oxygen has three main stable isotopes:
- Oxygen-16 (¹⁶O): This is the most abundant isotope, comprising about 99.76% of naturally occurring oxygen. It has 8 protons and 8 neutrons.
- Oxygen-17 (¹⁷O): A less common isotope with 8 protons and 9 neutrons.
- Oxygen-18 (¹⁸O): Another less common isotope with 8 protons and 10 neutrons.
It's important to remember that although these isotopes have different numbers of neutrons and therefore different masses, they all have the same number of protons – 8 – and therefore retain their oxygen identity. The differences in neutron count can influence certain physical properties, such as the density of water, but the fundamental chemical properties remain largely the same.
The Significance of Oxygen's Eight Protons
The presence of eight protons in the oxygen atom has profound implications for its chemical behavior and its role in various natural processes. This number dictates:
-
Electron Configuration: The eight protons attract eight electrons, leading to a specific electron configuration. Oxygen's electron configuration is 1s²2s²2p⁴. This configuration explains oxygen's high electronegativity, its tendency to readily attract electrons from other atoms.
-
Chemical Bonding: Oxygen's high electronegativity results in the formation of strong chemical bonds, particularly covalent bonds. This ability to form strong bonds is central to the role oxygen plays in various chemical reactions, including oxidation, combustion, and respiration. Oxygen's ability to form double bonds, crucial in many biological molecules, is also a direct consequence of its electron configuration.
-
Oxidation and Reduction: Oxygen's significant role in oxidation-reduction (redox) reactions is linked to its high electron affinity. It readily accepts electrons from other atoms, leading to the oxidation of those atoms and the reduction of oxygen itself. This process is vital in respiration, where oxygen acts as a terminal electron acceptor in the electron transport chain.
-
Biological Significance: The unique properties arising from its eight protons make oxygen essential for life as we know it. It's a key component of water (H₂O), a universal solvent and essential for many biological processes. Oxygen is also a vital component of organic molecules like carbohydrates, lipids, proteins, and nucleic acids, the building blocks of life.
Beyond the Basics: Applications and Further Exploration
The fundamental knowledge of oxygen's eight protons opens the door to understanding numerous applications and further explorations in various fields:
-
Medical Applications: Oxygen therapy is a critical medical intervention for individuals with respiratory problems. Understanding oxygen's chemical behavior is crucial for developing effective delivery methods and treatments.
-
Industrial Applications: Oxygen is widely used in various industries, including welding, metal fabrication, and chemical manufacturing. Its reactivity and ability to support combustion are key to these applications.
-
Environmental Science: Oxygen's role in the Earth's atmosphere and its impact on the environment are significant areas of study. Research on oxygen cycles and the effects of environmental changes on oxygen levels is crucial for understanding climate change and ecosystem health.
-
Space Exploration: The search for life beyond Earth often focuses on detecting the presence of oxygen as a potential biosignature. Understanding oxygen's properties is crucial for interpreting data from space missions.
Conclusion: The Importance of a Simple Number
The simple answer to the question "What is the number of protons in oxygen?" is 8. However, this seemingly simple number holds immense significance. It’s the foundation upon which oxygen's unique properties, chemical behavior, and biological importance are built. Understanding this fundamental aspect of oxygen's atomic structure provides a crucial stepping stone towards comprehending its vital role in the universe, from the processes within our bodies to the grand scheme of environmental and cosmological phenomena. The number 8, in the context of oxygen, is much more than just a numerical value; it's a key to unlocking a deeper understanding of the world around us.
Latest Posts
Latest Posts
-
Examples Of Essential And Nonessential Nutrients
Mar 17, 2025
-
Electric Potential From A Point Charge
Mar 17, 2025
-
Whats The Derivative Of A Constant
Mar 17, 2025
-
Differential Rate Law For Zero Order Reaction
Mar 17, 2025
-
Cell The Basic Unit Of Life
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Is The Number Of Protons In Oxygen . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
