What Is The Oxidation State Of Cl
Muz Play
Mar 18, 2025 · 6 min read
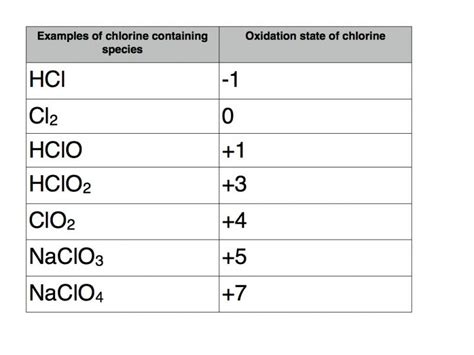
Table of Contents
What is the Oxidation State of Cl? A Comprehensive Guide
Chlorine (Cl), a highly reactive nonmetal, is renowned for its diverse oxidation states, making it a fascinating element to study in chemistry. Understanding its oxidation states is crucial for predicting its chemical behavior and its role in various compounds and reactions. This comprehensive guide delves deep into the oxidation states of chlorine, explaining the factors influencing them and showcasing examples in different chemical contexts.
Understanding Oxidation States
Before diving into the specific oxidation states of chlorine, let's establish a clear understanding of what oxidation state actually means. The oxidation state, also known as the oxidation number, represents the hypothetical charge an atom would have if all bonds to atoms of different elements were 100% ionic. It's a crucial concept for balancing redox reactions and predicting the reactivity of elements.
Key Considerations:
- Electronegativity: The oxidation state is heavily influenced by the electronegativity of the atom and the atoms it bonds with. Electronegativity is the ability of an atom to attract electrons towards itself in a chemical bond. Highly electronegative atoms tend to have negative oxidation states.
- Bond Type: The type of bond (ionic or covalent) plays a significant role. In ionic bonds, the oxidation state reflects the actual charge of the ion. In covalent bonds, the oxidation state is assigned based on a set of rules that allocate electrons to the more electronegative atom.
- Rules for Assigning Oxidation States: A set of established rules guides the assignment of oxidation states. These rules involve assigning specific oxidation states to certain elements (e.g., alkali metals are +1, alkaline earth metals are +2, etc.) and then balancing the overall charge of the molecule or ion.
Common Oxidation States of Chlorine
Chlorine exhibits a remarkable range of oxidation states, ranging from -1 to +7. This versatility arises from its electronic configuration ([Ne] 3s²3p⁵) and its ability to gain or lose electrons to achieve a stable octet.
1. -1 Oxidation State: This is the most common oxidation state for chlorine. It occurs when chlorine gains one electron to complete its octet, forming the chloride ion (Cl⁻). This state is prevalent in many ionic compounds like sodium chloride (NaCl), potassium chloride (KCl), and numerous other metal chlorides. This stability is due to the chloride ion's full valence shell.
Examples:
- NaCl (Sodium Chloride): Na⁺Cl⁻. Chlorine exhibits a -1 oxidation state.
- HCl (Hydrochloric Acid): While technically a covalent compound, chlorine's electronegativity is significantly higher than hydrogen, making its oxidation state -1.
- MgCl₂ (Magnesium Chloride): Mg²⁺(Cl⁻)₂. Chlorine again shows a -1 oxidation state.
2. +1 Oxidation State: Chlorine can also exist in the +1 oxidation state, typically found in compounds like hypochlorites. In these compounds, chlorine has lost one electron.
Examples:
- NaClO (Sodium Hypochlorite): Na⁺ClO⁻. The oxidation state of chlorine here is +1. This compound is commonly found in bleach.
3. +3 Oxidation State: This oxidation state is less common but is observed in chlorites.
Examples:
- NaClO₂ (Sodium Chlorite): Na⁺ClO₂⁻. Chlorine is in the +3 oxidation state. Chlorites are used as bleaching agents and disinfectants.
4. +5 Oxidation State: Chlorates represent chlorine in the +5 oxidation state.
Examples:
- KClO₃ (Potassium Chlorate): K⁺ClO₃⁻. Chlorine exhibits a +5 oxidation state. Potassium chlorate is used in matches and fireworks.
5. +7 Oxidation State: The highest oxidation state for chlorine is +7, found in perchlorates.
Examples:
- KClO₄ (Potassium Perchlorate): K⁺ClO₄⁻. Here, chlorine demonstrates its +7 oxidation state. Potassium perchlorate is used as an oxidizer in rocket propellant.
Factors Affecting Chlorine's Oxidation State
Several factors contribute to the variability in chlorine's oxidation state:
- The nature of the other atoms in the compound: The electronegativity difference between chlorine and the atoms it bonds with significantly influences its oxidation state. More electronegative atoms will tend to draw electrons away from chlorine, resulting in a more positive oxidation state. Less electronegative atoms will result in a more negative oxidation state or even a zero oxidation state in its elemental form (Cl₂).
- The overall charge of the molecule or ion: The sum of the oxidation states of all atoms in a neutral molecule must equal zero. For ions, the sum of the oxidation states must equal the charge of the ion. This constraint dictates the possible oxidation states of chlorine within a given compound.
- The reaction conditions: The oxidation state of chlorine can change depending on the reaction conditions, such as the presence of oxidizing or reducing agents.
Importance of Understanding Chlorine's Oxidation States
The ability of chlorine to exist in multiple oxidation states is key to its diverse applications:
- Disinfectants: The various oxychlorine species (hypochlorites, chlorites, chlorates, and perchlorates) are potent disinfectants due to their strong oxidizing properties, stemming from chlorine's ability to change its oxidation state.
- Bleaching Agents: Similar to disinfection, the oxidizing power of these species makes them effective bleaching agents.
- Oxidizers in Rocket Propellants: The high oxidation state of chlorine in perchlorates provides strong oxidizing power, crucial for rocket propulsion.
- Industrial Chemistry: Chlorine compounds are used extensively in various industrial processes, including the production of plastics, solvents, and other chemicals. Understanding oxidation states helps in controlling the reactivity and selecting appropriate reactants.
Determining the Oxidation State of Chlorine in a Compound
To determine the oxidation state of chlorine in a specific compound, follow these steps:
- Assign oxidation states to elements with fixed oxidation states: Start by assigning oxidation states to elements with predictable oxidation states (e.g., alkali metals = +1, alkaline earth metals = +2, oxygen = -2 (except in peroxides), hydrogen = +1).
- Let 'x' represent the oxidation state of chlorine: Use 'x' as a variable to represent the unknown oxidation state of chlorine.
- Apply the charge balance rule: The sum of the oxidation states of all atoms in a neutral molecule must equal zero. For ions, the sum of the oxidation states must equal the charge of the ion.
- Solve for 'x': Set up an algebraic equation based on the charge balance rule and solve for 'x' to determine the oxidation state of chlorine.
Example: Determine the oxidation state of chlorine in KClO₃.
- Potassium (K) has an oxidation state of +1.
- Oxygen (O) has an oxidation state of -2.
- Let x be the oxidation state of chlorine (Cl).
- The overall charge of KClO₃ is 0 (neutral molecule).
- Equation: (+1) + x + 3(-2) = 0
- Solving for x: x = +5. Therefore, the oxidation state of chlorine in KClO₃ is +5.
Conclusion
The oxidation state of chlorine is a multifaceted topic with significant implications for understanding its chemical behavior and its diverse applications across various fields. Its ability to exist in a broad range of oxidation states, from -1 to +7, highlights its remarkable versatility. Mastering the concept of oxidation states and the factors influencing them is essential for anyone studying chemistry, particularly in the context of redox reactions and the properties of chlorine compounds. By understanding these principles, we can better predict reactivity, design chemical processes, and appreciate the multifaceted role of chlorine in our world.
Latest Posts
Latest Posts
-
Is Ductile A Metal Or Nonmetal
Mar 18, 2025
-
Queremos Tus Palabras Oi Oyo Oyendo Oir
Mar 18, 2025
-
How To Find Profit Maximizing Price
Mar 18, 2025
-
Which One Increases Number Of Collisions Between The Reactants
Mar 18, 2025
-
Which Part Of The Coxal Bone Articulates With The Sacrum
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about What Is The Oxidation State Of Cl . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
