Which One Increases Number Of Collisions Between The Reactants
Muz Play
Mar 18, 2025 · 5 min read
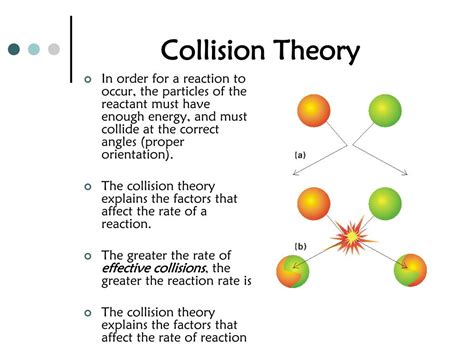
Table of Contents
Which Factor Increases the Number of Collisions Between Reactants? Maximizing Reaction Rates Through Collision Theory
Understanding chemical reaction rates is fundamental to chemistry. The speed at which reactants transform into products dictates everything from industrial processes to biological functions. At the heart of reaction rate lies collision theory, which postulates that for a reaction to occur, reactant particles must collide with sufficient energy and proper orientation. This article delves into the factors that influence the frequency and effectiveness of these collisions, focusing primarily on those that directly increase the number of collisions between reactants.
The Fundamental Role of Collisions in Chemical Reactions
Before exploring the factors that increase collision frequency, let's establish the core principle: the more collisions, the faster the reaction. A chemical reaction, at its most basic level, involves the rearrangement of atoms and molecules. This rearrangement can only happen when reactant particles physically interact – they must collide. However, not all collisions are created equal. A collision must meet two criteria:
-
Sufficient Energy (Activation Energy): Reactant particles must possess a minimum amount of kinetic energy, known as the activation energy (Ea), to overcome the energy barrier that prevents the reaction from proceeding. This energy is needed to break existing bonds and initiate the formation of new ones.
-
Correct Orientation: Even with sufficient energy, the collision must occur with the correct orientation for the reactive parts of the molecules to interact effectively. If the molecules collide in an unfavorable orientation, the collision will be ineffective, and no reaction will occur.
This article focuses primarily on increasing the number of collisions, while acknowledging that increasing the effectiveness of collisions (by increasing energy and improving orientation) also accelerates the reaction rate.
Factors that Increase the Number of Collisions Between Reactants
Several factors can significantly increase the number of collisions between reactant particles, thus speeding up the reaction rate. Let's examine each in detail:
1. Increasing the Concentration of Reactants
Higher concentration means more particles in a given volume. This directly translates to a higher probability of collisions occurring. Imagine a crowded room – the chances of two people bumping into each other are much higher than in an empty room. Similarly, increasing the concentration of reactants increases the likelihood of reactant particles colliding. This is a fundamental principle in reaction kinetics. Doubling the concentration of one reactant (assuming all other factors remain constant) roughly doubles the reaction rate.
2. Increasing the Surface Area of Solid Reactants
When one or more reactants are solids, the surface area available for interaction significantly impacts the collision frequency. A larger surface area exposes more reactant particles to potential collisions. Consider a reaction involving a solid reactant: a finely powdered solid will react much faster than a single, large lump of the same solid. This is because the powder has a vastly larger surface area, providing more opportunities for collisions with other reactants. Techniques like grinding or using catalysts with high surface area are employed to maximize this effect.
3. Increasing the Temperature of the Reaction Mixture
Temperature is a crucial factor influencing reaction rates. Higher temperatures impart greater kinetic energy to the reactant particles. This increased kinetic energy results in faster particle movement and more frequent, energetic collisions. While higher temperatures also increase the effectiveness of collisions by providing more particles with sufficient activation energy, the increased collision frequency itself is a significant contributor to the overall rate increase. The effect of temperature on reaction rate is typically described using the Arrhenius equation.
4. Stirring or Agitation of the Reaction Mixture
In reactions involving liquids or solutions, stirring or agitation plays a crucial role in increasing the number of collisions. Stirring ensures that reactants are evenly distributed and constantly moving, preventing the formation of local areas with depleted reactant concentrations. Without stirring, the reaction might be limited by the diffusion of reactants into each other, significantly reducing the collision frequency.
5. Using a Catalyst
Catalysts are substances that speed up chemical reactions without being consumed themselves. While catalysts primarily act by lowering the activation energy (thus increasing the effectiveness of collisions), they can also indirectly influence the number of collisions. Heterogeneous catalysts, particularly those with high surface areas, provide a surface for reactant molecules to adsorb onto, increasing their local concentration and hence the probability of collision. This localized increase in concentration acts as a collision-enhancing factor.
Collision Theory vs. Other Reaction Rate Theories
While collision theory provides a valuable framework for understanding reaction rates, it's crucial to acknowledge its limitations and the existence of other theoretical models. Collision theory is primarily applicable to reactions in the gas phase or homogeneous solutions. For more complex reactions or reactions involving heterogeneous catalysts, other theories, such as transition state theory, provide a more comprehensive understanding. However, the fundamental principle of collisions as a prerequisite for reaction remains central to all these theories.
Practical Applications of Increasing Collision Frequency
Understanding how to manipulate collision frequency has countless practical applications across various fields:
- Industrial Chemical Processes: Optimizing reaction conditions (temperature, pressure, concentration, stirring) to maximize reaction rates is vital for efficient industrial production.
- Pharmaceutical Drug Development: Understanding reaction kinetics is crucial in designing efficient syntheses for new drugs, often relying on catalysts and optimized reaction conditions.
- Environmental Chemistry: Understanding reaction rates helps predict the fate of pollutants in the environment and develop strategies for remediation.
- Food Science: The preservation of food relies on controlling reaction rates to slow down spoilage processes.
- Biological Systems: Enzyme catalysis in biological systems relies on efficient collision between enzymes and substrates. Understanding how these collisions are optimized is crucial for understanding biological processes.
Conclusion: Optimizing Collisions for Enhanced Reaction Rates
Increasing the number of collisions between reactant molecules is a powerful strategy for accelerating chemical reactions. By manipulating factors such as concentration, surface area, temperature, stirring, and catalyst usage, we can significantly influence the reaction rate. While increasing the effectiveness of collisions (sufficient energy and proper orientation) is equally crucial, maximizing collision frequency remains a foundational aspect of controlling reaction rates and optimizing various chemical processes across different fields. The principles of collision theory offer a powerful lens through which we can understand and manipulate the dynamic world of chemical reactions. Further research into enhancing both collision frequency and effectiveness continues to be a major focus in chemistry, leading to advancements in diverse areas from materials science to medicine.
Latest Posts
Latest Posts
-
A Virus That Undergoes Lysogeny Is A An
Mar 18, 2025
-
Where Are Metals Located In The Periodic Table
Mar 18, 2025
-
Gay Lussac Law Of Combining Volume
Mar 18, 2025
-
How To Analysis Mass Spectrometry Data
Mar 18, 2025
-
Reaction Of Ester With Lithium Aluminium Hydride
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Which One Increases Number Of Collisions Between The Reactants . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
