Where Are Metals Located In The Periodic Table
Muz Play
Mar 18, 2025 · 6 min read
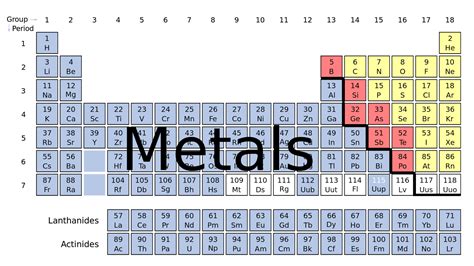
Table of Contents
Where Are Metals Located in the Periodic Table? A Comprehensive Guide
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. Understanding the arrangement of elements allows us to predict their behavior and reactivity. One of the most fundamental classifications within the periodic table is the division into metals, nonmetals, and metalloids. This article delves deep into the location of metals within the periodic table, exploring their characteristics and exceptions.
The Broad Sweep: Metals Dominate the Left
The vast majority of metals reside on the left-hand side and in the center of the periodic table. This isn't a rigid line, but rather a gradual transition zone. You'll find a clear majority of metallic elements in groups 1 (alkali metals), 2 (alkaline earth metals), and the transition metal blocks (groups 3-12). The lanthanides and actinides, positioned separately at the bottom, are also entirely composed of metals. This dominant presence highlights the prevalence of metals in the natural world and their importance in various applications.
Understanding the Electron Configuration: The Key to Metallic Properties
The location of metals is directly tied to their electron configurations. Metals generally have few valence electrons (electrons in the outermost shell). This characteristic allows them to readily lose these electrons, forming positive ions (cations). This electron-losing tendency is the foundation of many metallic properties, including:
- Conductivity: The loosely held valence electrons are free to move throughout the metal lattice, facilitating the flow of electrical current and heat.
- Malleability and Ductility: The ability to be hammered into shapes (malleability) and drawn into wires (ductility) stems from the ability of metal atoms to slide past each other without disrupting the metallic bonding.
- Luster: The characteristic shine of metals arises from the interaction of light with the delocalized electrons.
- High Density: Metals generally possess a relatively high density compared to nonmetals due to their close-packed atomic arrangements.
Delving Deeper: Specific Metal Groups and Their Locations
Let's explore the specific locations and characteristics of different metal groups within the periodic table:
1. Alkali Metals (Group 1): The Most Reactive Metals
Found in the first column of the periodic table, alkali metals are extremely reactive. They possess only one valence electron, which they readily lose to form +1 ions. Their reactivity increases as you go down the group. This high reactivity makes them unsuitable for many everyday applications in their pure form, but their compounds are essential in various industries and biological processes.
2. Alkaline Earth Metals (Group 2): Less Reactive, Still Important
Located in the second column, alkaline earth metals have two valence electrons, making them less reactive than alkali metals but still highly reactive compared to other elements. They typically form +2 ions. These metals, though less reactive than their alkali counterparts, find applications in various materials and technologies. Magnesium, for instance, is a key component of lightweight alloys.
3. Transition Metals (Groups 3-12): A Diverse Group with Varied Properties
The transition metals occupy the central block of the periodic table. Their defining characteristic is the presence of partially filled d orbitals. This feature leads to a diverse range of properties, including multiple oxidation states, formation of colored compounds, and catalytic activity. Transition metals are crucial in countless applications, from construction materials (iron, steel) to electronics (copper, gold).
4. Lanthanides and Actinides: The Inner Transition Metals
These two series, positioned separately at the bottom of the periodic table, are collectively known as the inner transition metals. They are characterized by the filling of the 4f (lanthanides) and 5f (actinides) orbitals. Many of these elements are radioactive, and their properties are often similar within each series due to the filling of the inner f orbitals. Their applications span nuclear technology and specialized materials science.
The Fuzzy Boundaries: Metalloids and the Transition Zone
The classification of elements isn't always clear-cut. There's a transition zone between metals and nonmetals where elements exhibit properties of both. These elements are called metalloids, and they are typically found along a stair-step line separating metals and nonmetals on the periodic table. Boron, silicon, germanium, arsenic, antimony, tellurium, and polonium are generally considered metalloids. Their properties vary significantly, depending on the specific element and its conditions. For example, silicon, a metalloid, is a crucial component of semiconductors.
Notable Exceptions: Non-Metallic Behavior in Metals
While the majority of elements on the left and center of the periodic table are metals, there are some exceptions. Certain elements exhibit atypical behavior, showcasing characteristics more aligned with nonmetals. This is often due to factors like:
- Small atomic size: Smaller atoms can have stronger electronegativity, influencing their bonding behavior.
- High ionization energy: Some metals require more energy to lose electrons, reducing their metallic character.
- Special electronic configurations: Unique electronic arrangements can modify an element's behavior, leading to less typical metallic properties.
This demonstrates that the periodic table, while providing a strong framework for understanding element classification, is not an absolute guide. The nuances of chemical behavior always require careful consideration.
Practical Applications: The Ubiquity of Metals
Metals' unique properties have led to their widespread use across numerous industries and aspects of daily life. Here are some examples:
- Construction: Iron, steel, aluminum, and other metals are the backbone of construction, providing structural strength and durability.
- Transportation: Aluminum, steel, and various alloys are essential in the automotive and aerospace industries for their lightweight and strength properties.
- Electronics: Copper, gold, silver, and other metals are crucial components in electronic devices for their conductivity and other electrical properties.
- Medicine: Many metals and their compounds are utilized in medical applications, such as implants, contrast agents, and drug delivery systems.
- Energy: Various metals are used in energy generation and storage, including batteries, fuel cells, and nuclear reactors.
Conclusion: The Periodic Table as a Roadmap to Metallic Properties
The location of metals within the periodic table provides a valuable roadmap to understanding their properties and behaviors. While the majority of metals are clearly located on the left and in the center, the transition zone between metals and nonmetals highlights the complexities of chemical behavior. Understanding the relationship between electron configuration and metallic properties is crucial for predicting and explaining the diverse applications of these elements in various fields. Further exploration of the periodic table's organization and the specific properties of each metal group enhances our grasp of chemistry and its impact on the world around us. The periodic table serves not merely as a list of elements, but as a powerful tool for predicting and explaining the behavior of matter. This fundamental understanding allows for advancements in material science, engineering, and various other scientific disciplines.
Latest Posts
Latest Posts
-
An Intermediate Phenotype Indicates That A Trait Has Dominance
Mar 19, 2025
-
Plant Is Where Photosynthesis Takes Place
Mar 19, 2025
-
The Force Driving Plate Tectonics Is
Mar 19, 2025
-
Converting Double Integrals To Polar Coordinates
Mar 19, 2025
-
How Do You Convert Moles To Volume
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Where Are Metals Located In The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
