What Is The Product Of The Hydrogenation Of An Alkene
Muz Play
Mar 26, 2025 · 6 min read
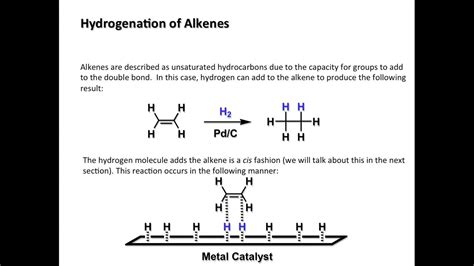
Table of Contents
What is the Product of the Hydrogenation of an Alkene?
The hydrogenation of alkenes is a fundamental reaction in organic chemistry, offering a straightforward method to synthesize alkanes. Understanding this reaction, its mechanism, and the resulting products is crucial for any aspiring chemist. This comprehensive guide delves into the intricacies of alkene hydrogenation, explaining the process, the factors influencing it, and its widespread applications.
Understanding Alkenes and Alkanes
Before diving into the hydrogenation process, let's briefly review the characteristics of alkenes and alkanes.
Alkenes: The Unsaturated Hydrocarbons
Alkenes are hydrocarbons containing at least one carbon-carbon double bond (C=C). This double bond introduces unsaturation into the molecule, making alkenes more reactive than their saturated counterparts, the alkanes. The presence of the pi (π) bond in the double bond is the key to their reactivity. This electron-rich region is susceptible to electrophilic attack. Simple examples include ethene (C₂H₄), propene (C₃H₆), and butene (C₄H₈). The presence of the double bond also leads to isomerism, with alkenes exhibiting cis-trans (or E/Z) isomerism depending on the substituents around the double bond.
Alkanes: The Saturated Hydrocarbons
Alkanes are saturated hydrocarbons, meaning they contain only single carbon-carbon bonds (C-C). They are relatively unreactive compared to alkenes due to the absence of the reactive pi bond. Alkanes are the simplest class of organic compounds, and their properties generally depend on the length of the carbon chain. Examples include methane (CH₄), ethane (C₂H₆), and propane (C₃H₈).
The Hydrogenation Reaction: Adding Hydrogen Across the Double Bond
Hydrogenation is a chemical process that involves the addition of hydrogen (H₂) to a molecule, usually in the presence of a catalyst. In the context of alkenes, hydrogenation results in the saturation of the double bond, converting the alkene into an alkane. The general reaction can be represented as:
R₁CH=CHR₂ + H₂ → R₁CH₂-CH₂R₂
Where R₁ and R₂ represent alkyl groups (or hydrogen atoms).
The reaction fundamentally involves the breaking of the pi bond in the alkene and the formation of two new sigma (σ) bonds with the hydrogen atoms. This results in a significant change in the molecule's properties, transforming the unsaturated alkene into a saturated alkane.
The Role of Catalysts in Alkene Hydrogenation
The hydrogenation of alkenes typically requires a catalyst to proceed at a reasonable rate. These catalysts facilitate the reaction by providing a surface for the reactants to adsorb, weakening the H-H bond and the C=C bond, thereby lowering the activation energy of the reaction.
Common catalysts include:
- Platinum (Pt): A highly effective catalyst, often used in the form of platinum oxide (PtO₂) which is reduced in situ to platinum metal.
- Palladium (Pd): Another highly active catalyst, often used as palladium on charcoal (Pd/C).
- Nickel (Ni): A less expensive and readily available catalyst, often used as Raney nickel.
The choice of catalyst can influence the reaction rate and selectivity, particularly in the hydrogenation of complex alkenes with multiple double bonds.
The Mechanism of Catalytic Hydrogenation
The mechanism of catalytic hydrogenation is generally accepted to be a heterogeneous process, occurring on the surface of the catalyst. A simplified description involves the following steps:
- Adsorption: Both the alkene and hydrogen molecules adsorb onto the catalyst surface.
- Activation: The adsorbed molecules are activated, weakening the bonds within them (C=C and H-H).
- Addition: Hydrogen atoms add across the double bond of the alkene, forming two new C-H bonds.
- Desorption: The resulting alkane molecule desorbs from the catalyst surface.
This cycle repeats, allowing for the continuous hydrogenation of alkene molecules. The specific details of the mechanism can be complex and vary depending on the catalyst used.
Factors Affecting Hydrogenation
Several factors influence the rate and efficiency of alkene hydrogenation:
- Catalyst: The type and amount of catalyst significantly impact the reaction rate. More active catalysts, like platinum, generally lead to faster reactions.
- Temperature: Higher temperatures generally increase the rate of hydrogenation, providing more kinetic energy to overcome the activation energy barrier.
- Pressure: Increasing the hydrogen pressure increases the concentration of hydrogen at the catalyst surface, leading to a faster reaction rate.
- Solvent: The solvent used can affect the solubility of the alkene and hydrogen, influencing the rate of reaction. Often, non-polar solvents like ethanol or hexane are used.
- Steric Hindrance: Bulky substituents around the double bond can hinder the approach of the hydrogen molecule to the catalyst surface, slowing down the reaction.
Applications of Alkene Hydrogenation
The hydrogenation of alkenes is a widely used reaction with numerous applications in various industries:
- Food Industry: Hydrogenation is used extensively in the food industry to convert unsaturated vegetable oils into saturated fats, such as margarine. This process increases the shelf life and changes the physical properties of the oils.
- Pharmaceutical Industry: Hydrogenation is a crucial step in the synthesis of many pharmaceuticals, where it's used to selectively reduce double bonds in complex molecules.
- Petrochemical Industry: The process is used to saturate unsaturated hydrocarbons present in petroleum refining, improving the quality and properties of fuels.
- Chemical Industry: Hydrogenation is used to produce various chemicals, including alcohols, amines, and other saturated compounds.
Predicting Products: A Practical Approach
Predicting the product of alkene hydrogenation is relatively straightforward. The double bond is simply replaced by two single bonds, each bonded to a hydrogen atom. For example:
- Ethene (C₂H₄) hydrogenation: Produces ethane (C₂H₆).
- Propene (C₃H₆) hydrogenation: Produces propane (C₃H₈).
- 1-butene (CH₂=CHCH₂CH₃) hydrogenation: Produces butane (CH₃CH₂CH₂CH₃).
- Cyclohexene hydrogenation: Produces cyclohexane.
This applies regardless of the presence of other functional groups in the molecule, although these groups might influence the reaction conditions required.
Dealing with Multiple Double Bonds: Partial vs. Complete Hydrogenation
Alkenes with multiple double bonds (polyenes) can undergo hydrogenation at each double bond. The extent of hydrogenation depends on the reaction conditions. With excess hydrogen and a sufficiently active catalyst, complete hydrogenation occurs, saturating all double bonds. However, under milder conditions (less hydrogen or less active catalysts), partial hydrogenation can occur, resulting in the formation of intermediate products with only some of the double bonds saturated. Careful control of reaction conditions allows chemists to selectively hydrogenate specific double bonds in complex molecules.
Beyond Simple Alkenes: Hydrogenation of More Complex Molecules
The principle of hydrogenation remains the same even when dealing with more complex molecules containing double bonds as part of larger functional groups. For example, hydrogenation can be applied to:
- Aldehydes and Ketones: While not strictly alkene hydrogenation, the carbonyl group (C=O) can be reduced to an alcohol (-OH) using hydrogenation methods. This usually requires different catalysts compared to those used for alkenes.
- Alkynes: Alkynes (with a triple bond, C≡C) can also be hydrogenated, potentially in a stepwise fashion to first yield an alkene and then an alkane.
- Aromatic Compounds: Aromatic compounds, while possessing double bonds in a conjugated ring system, require more strenuous conditions for complete hydrogenation.
Conclusion
The hydrogenation of alkenes is a fundamental reaction in organic chemistry, providing a versatile method for converting unsaturated compounds to their saturated counterparts. By understanding the reaction mechanism, the influence of various factors, and the applications of this reaction, chemists can effectively utilize this powerful tool in the synthesis of a wide range of organic molecules. The predictability of the product coupled with the wide range of application makes alkene hydrogenation an indispensable tool in many scientific fields. The ability to control reaction conditions allows for both complete and partial hydrogenation, opening up a spectrum of synthetic possibilities. Further exploration into the nuances of this reaction promises ongoing advances and innovations in organic synthesis and beyond.
Latest Posts
Latest Posts
-
What Are The Characteristics Of Liquid
Mar 29, 2025
-
Are Covalent Bonds Strong Or Weak
Mar 29, 2025
-
The Horizontal Transfer Process Known As Transduction
Mar 29, 2025
-
Write The Relation As A Set Of Ordered Pairs
Mar 29, 2025
-
Us History Reconstruction To The Present
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about What Is The Product Of The Hydrogenation Of An Alkene . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
