Are Covalent Bonds Strong Or Weak
Muz Play
Mar 29, 2025 · 6 min read
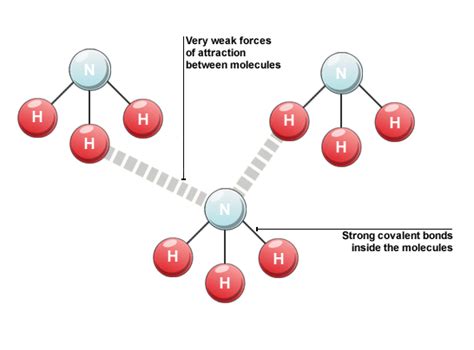
Table of Contents
Are Covalent Bonds Strong or Weak? It's Complicated!
The question of whether covalent bonds are strong or weak isn't a simple yes or no answer. The strength of a covalent bond is highly dependent on several factors, making it a nuanced topic requiring a deeper exploration. While generally considered strong compared to intermolecular forces, the strength varies dramatically depending on the atoms involved and the specific conditions. This article will delve into the intricacies of covalent bond strength, examining the factors that influence it and offering examples to illustrate the complexities involved.
Understanding Covalent Bonds: A Quick Recap
Before diving into the strength debate, let's briefly review the fundamentals of covalent bonding. Covalent bonds form when two atoms share one or more pairs of electrons to achieve a more stable electron configuration, often resembling a noble gas. This sharing results in a strong electrostatic attraction between the positively charged nuclei and the negatively charged shared electrons. This attraction holds the atoms together, forming a molecule. Unlike ionic bonds, which involve the complete transfer of electrons, covalent bonds feature a more even distribution (though not necessarily equal) of electron density between the atoms.
Factors Influencing Covalent Bond Strength
Several key factors determine the strength of a covalent bond. These include:
1. Atomic Size and Electronegativity
Smaller atoms generally form stronger covalent bonds. This is because the shared electrons are closer to the positively charged nuclei, leading to a stronger electrostatic attraction. Similarly, the electronegativity of the atoms plays a crucial role. Electronegativity is a measure of an atom's ability to attract electrons towards itself in a chemical bond. A large difference in electronegativity between two atoms results in a polar covalent bond, where the electrons are unevenly shared. While this doesn't necessarily make the bond stronger in terms of pure energy, it can increase the overall stability of the molecule due to dipole-dipole interactions. However, an extremely large difference in electronegativity leads to ionic bonding rather than a strong covalent bond.
Example: Consider the C-H bond versus the O-H bond. Oxygen is significantly more electronegative than carbon, resulting in a stronger O-H bond than a C-H bond because of the greater attraction of the shared electrons towards the oxygen atom.
2. Bond Order and Bond Length
Bond order refers to the number of electron pairs shared between two atoms. A higher bond order indicates a stronger bond because more electrons are shared, leading to a greater electrostatic attraction. Conversely, bond length, the distance between the nuclei of the two bonded atoms, is inversely proportional to bond strength. Shorter bonds are stronger because the nuclei are closer together, leading to increased electrostatic attraction.
Example: A triple bond (bond order = 3), such as in nitrogen gas (N≡N), is stronger and shorter than a double bond (bond order = 2), such as in oxygen gas (O=O), which is stronger and shorter than a single bond (bond order = 1), such as in hydrogen gas (H-H).
3. Resonance Structures
In some molecules, the electron density is delocalized across multiple bonds, a phenomenon known as resonance. Resonance structures contribute to increased bond strength and stability because the electrons are distributed over a larger region, effectively strengthening the overall bond order.
Example: Benzene (C₆H₆) exhibits resonance, with the electrons delocalized over the six carbon atoms, resulting in a stronger and more stable molecule than a hypothetical structure with alternating single and double bonds.
4. Hybridization
Hybridization is the mixing of atomic orbitals to form new hybrid orbitals with different shapes and energies. The type of hybridization affects the bond strength and geometry of the molecule. For example, sp hybridized orbitals are shorter and stronger than sp² and sp³ hybridized orbitals because of their increased s-character.
Example: A carbon-carbon triple bond (sp hybridization) is stronger than a carbon-carbon double bond (sp² hybridization), which is stronger than a carbon-carbon single bond (sp³ hybridization).
5. Steric Hindrance
Steric hindrance refers to the repulsive forces between atoms or groups of atoms that are close together in a molecule. If bulky groups are attached to the atoms forming a covalent bond, these steric interactions can weaken the bond by increasing the bond length and reducing the overlap between the atomic orbitals.
Example: In very large organic molecules, steric hindrance can significantly affect the strength of bonds, making them weaker than they would be in smaller, less crowded molecules.
Comparing Covalent Bond Strengths: A Quantitative Perspective
While qualitative comparisons based on the factors above are useful, it's important to understand that bond strengths are quantifiable. Bond dissociation energy (BDE) is a measure of the energy required to break a specific covalent bond. This energy is typically expressed in kJ/mol (kilojoules per mole). Higher BDE values indicate stronger bonds. It's crucial to remember that BDE values are highly specific to the particular bond and the conditions under which it's measured (temperature, pressure, etc.).
Covalent Bonds: Strong in Context
While some covalent bonds are relatively weak compared to others, it's vital to understand their strength relative to other types of chemical interactions. Covalent bonds are significantly stronger than intermolecular forces like van der Waals forces, hydrogen bonds, and dipole-dipole interactions. These intermolecular forces are responsible for many physical properties of molecules, such as boiling point and melting point, but they are significantly weaker than the covalent bonds that hold the atoms within a molecule together.
Examples Illustrating the Range of Covalent Bond Strengths
To further illustrate the variability of covalent bond strength, let's consider some examples:
-
Strong Covalent Bonds: The triple bond in nitrogen gas (N≡N) has a very high BDE (945 kJ/mol), making it incredibly stable. Similarly, carbon-carbon triple bonds are extremely strong.
-
Moderate Covalent Bonds: The C-C single bond (347 kJ/mol) is a relatively strong bond, essential for the structure of organic molecules. The O-H bond (463 kJ/mol) is another example of a moderate strength bond, crucial for many biological systems.
-
Weaker Covalent Bonds: While still stronger than intermolecular forces, some covalent bonds are relatively weaker. For instance, certain bonds involving halogens can be relatively weak compared to bonds between atoms with smaller atomic radii.
Conclusion: Strength is Relative
The strength of a covalent bond is not an absolute property. It’s a complex interplay of atomic properties, bond order, resonance, and steric effects. While generally stronger than intermolecular forces, covalent bonds exhibit a wide range of strengths, impacting the properties and reactivity of molecules across the chemical spectrum. Understanding these nuances is key to comprehending the behavior of molecules in various contexts. Further research into specific bond types and their respective BDE values provides a deeper understanding of the fascinating world of chemical bonding.
Latest Posts
Latest Posts
-
Where Is Genetic Information Of The Cell Stored
Apr 01, 2025
-
The Amount Of Matter In An Object Is
Apr 01, 2025
-
What Is The Amdr For Fat For Adults
Apr 01, 2025
-
What Is The Difference Between A Closed And Open System
Apr 01, 2025
-
What Is The Charge Of Sodium
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Are Covalent Bonds Strong Or Weak . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
