What Is The Charge Of Sodium
Muz Play
Apr 01, 2025 · 6 min read
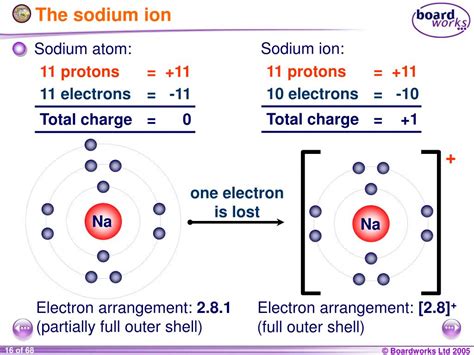
Table of Contents
What is the Charge of Sodium? Understanding Sodium's Ionic Behavior
Sodium (Na), an alkali metal found abundantly in nature, plays a crucial role in various biological and chemical processes. Understanding its charge is fundamental to grasping its behavior in these processes. This comprehensive guide dives deep into the charge of sodium, explaining its ionic nature, its interactions with other elements, and its significance in different fields.
The Basics: Sodium's Electronic Structure and Ionization
To understand the charge of sodium, we must first examine its electronic structure. Sodium has an atomic number of 11, meaning it possesses 11 protons in its nucleus and 11 electrons orbiting the nucleus. These electrons are arranged in energy levels or shells. The electronic configuration of sodium is 2, 8, 1. This means it has two electrons in the first shell, eight in the second shell, and one lone electron in its outermost shell, also known as the valence shell.
Valence electrons determine an atom's chemical reactivity. Sodium's single valence electron is loosely bound to the nucleus. This makes it relatively easy for sodium to lose this electron to achieve a stable electron configuration. This process is called ionization.
When sodium loses its single valence electron, it becomes a positively charged ion, denoted as Na⁺. This positive charge results from the imbalance between the number of protons (11) and electrons (now 10). This process is energetically favorable for sodium because it achieves a stable octet configuration, mimicking the electron configuration of the noble gas neon (Ne), which is highly stable.
The Octet Rule and Sodium's Stability
The octet rule states that atoms tend to gain, lose, or share electrons to achieve a full outer shell of eight electrons. Sodium, by losing its single valence electron, achieves a stable electron configuration with eight electrons in its outermost shell (the second shell). This makes the sodium ion (Na⁺) significantly more stable than the neutral sodium atom (Na).
Sodium's Ionic Behavior: Formation of Ionic Compounds
The positively charged sodium ion (Na⁺) readily interacts with negatively charged ions to form ionic compounds. These compounds are held together by strong electrostatic forces of attraction between the positively charged cations (like Na⁺) and negatively charged anions.
A classic example is the formation of sodium chloride (NaCl), commonly known as table salt. Chlorine (Cl) has seven valence electrons and readily gains one electron to achieve a stable octet configuration, forming a negatively charged chloride ion (Cl⁻). The electrostatic attraction between the Na⁺ ion and the Cl⁻ ion forms the strong ionic bond in NaCl.
Other Ionic Compounds of Sodium
Sodium forms various ionic compounds with other elements, including:
- Sodium hydroxide (NaOH): A strong base used in various industrial applications.
- Sodium carbonate (Na₂CO₃): Used in glassmaking, detergents, and water softening.
- Sodium bicarbonate (NaHCO₃): A common baking ingredient and antacid.
- Sodium sulfate (Na₂SO₄): Used in the paper industry and as a laxative.
These compounds all exhibit the characteristic properties of ionic compounds, such as high melting and boiling points, solubility in water, and the ability to conduct electricity when dissolved in water or molten.
The Significance of Sodium's Charge in Biology
Sodium's +1 charge is absolutely critical in numerous biological processes. The movement of sodium ions across cell membranes is essential for:
- Nerve impulse transmission: The generation and propagation of nerve impulses depend on the controlled movement of sodium ions across neuronal cell membranes. This process involves the opening and closing of voltage-gated sodium channels.
- Muscle contraction: Similar to nerve impulse transmission, muscle contraction relies on the influx of sodium ions into muscle cells. This triggers a cascade of events leading to muscle fiber shortening.
- Fluid balance: Sodium ions play a vital role in maintaining the proper balance of fluids within and outside cells. This is crucial for maintaining blood pressure and overall bodily functions.
- Nutrient absorption: Sodium ions aid in the absorption of certain nutrients in the digestive system.
Imbalances in sodium levels can lead to severe health problems, including dehydration, muscle cramps, and even cardiac arrest.
Sodium's Role in Osmosis and Membrane Potential
The movement of sodium ions across cell membranes is closely linked to the process of osmosis, the movement of water across a semi-permeable membrane to equalize solute concentration. Sodium ions contribute significantly to the osmotic pressure within and outside cells.
Moreover, the unequal distribution of sodium ions across cell membranes contributes to the membrane potential, the electrical potential difference across the cell membrane. This membrane potential is essential for various cellular functions, including nerve impulse transmission and muscle contraction.
Sodium's Charge in Chemistry: Applications and Reactions
Sodium's positive charge influences its reactivity and its applications in various chemical processes.
Sodium in Chemical Reactions
Due to its strong tendency to lose an electron and form a positive ion, sodium is highly reactive, particularly with water and halogens. The reaction of sodium with water is exothermic, releasing significant heat and producing hydrogen gas and sodium hydroxide. Reactions with halogens (like chlorine) produce ionic salts such as sodium chloride.
Industrial Applications of Sodium Compounds
The ionic nature of sodium compounds makes them useful in various industrial processes. Sodium hydroxide, for example, is a crucial component in soap manufacturing, paper production, and the textile industry. Sodium carbonate is used in glass production, detergents, and water treatment.
Analytical Chemistry and Sodium Detection
The characteristic properties of sodium ions allow for their detection and quantification using various analytical techniques. Flame tests, for example, can detect sodium ions based on the characteristic yellow color they emit when heated in a flame. Other techniques, such as atomic absorption spectroscopy and ion chromatography, can precisely determine the concentration of sodium ions in various samples.
Beyond the Basics: Isotopes of Sodium and Nuclear Applications
While the common isotope of sodium (²³Na) has 11 protons and 12 neutrons, there are also radioactive isotopes of sodium, such as ²²Na, which are used in various applications:
- Medical imaging: Radioactive sodium isotopes can be used as tracers in medical imaging techniques such as PET (positron emission tomography) scans. These isotopes allow for the visualization of various physiological processes within the body.
- Industrial tracing: Radioactive sodium isotopes can be used to trace the flow of fluids in industrial processes, aiding in the optimization of various systems.
Conclusion: The Ubiquitous Importance of Sodium's Charge
The +1 charge of sodium is not merely a simple fact; it's a fundamental property that dictates its behavior and makes it a vital element in diverse fields. From the intricate processes within our bodies to its widespread industrial applications, understanding sodium's ionic behavior and its positive charge is crucial to comprehending its significance in the world around us. The information discussed here provides a solid foundation for further exploration into the fascinating chemistry and biological roles of this ubiquitous element. Further research into specific applications or biological processes involving sodium will only enhance your understanding of its pervasive importance.
Latest Posts
Latest Posts
-
Inscribed Circle In A Right Triangle
Apr 02, 2025
-
Bacteria And Archaea Are Both Domains Consisting Of Prokaryotic Organisms
Apr 02, 2025
-
What Organelles Do Plants Have That Animals Dont
Apr 02, 2025
-
Select The Five Major Mechanisms Of Antimicrobial Resistance
Apr 02, 2025
-
What Are The Three Characteristics Of All Metals
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Is The Charge Of Sodium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
