What Is The Smallest Part Of An Atom
Muz Play
Mar 28, 2025 · 6 min read
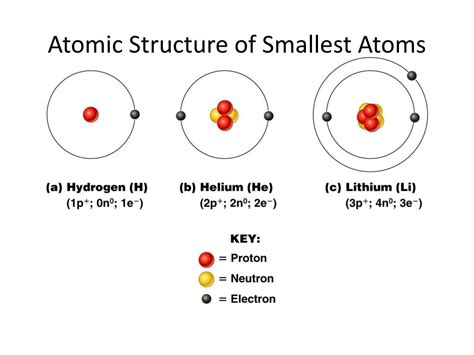
Table of Contents
What is the Smallest Part of an Atom? Delving into the Subatomic World
The atom, once considered the fundamental, indivisible building block of matter, has been revealed through centuries of scientific inquiry to be a complex system teeming with even smaller constituents. The question, "What is the smallest part of an atom?" doesn't have a simple, straightforward answer. It depends on how you define "part" and the level of detail you want to consider. Let's embark on a journey into the subatomic realm to explore this fascinating question.
The Historical Context: From Indivisibility to Complexity
The concept of the atom itself has evolved dramatically. Ancient Greek philosophers, notably Democritus and Leucippus, proposed the idea of atomos, meaning "indivisible," as the fundamental unit of matter. This was a philosophical concept, lacking the experimental evidence that would later revolutionize our understanding.
The true scientific exploration began in the late 19th and early 20th centuries with the groundbreaking work of scientists like J.J. Thomson, Ernest Rutherford, and Niels Bohr. Thomson's discovery of the electron, a negatively charged particle, shattered the notion of atomic indivisibility. Rutherford's gold foil experiment further unveiled the atom's internal structure, revealing a dense, positively charged nucleus surrounded by orbiting electrons. Bohr's model improved upon this by introducing quantized energy levels for electrons, paving the way for a more complete picture.
The Major Subatomic Particles: Protons, Neutrons, and Electrons
At a basic level, the atom is composed of three main subatomic particles:
1. Protons: The Positively Charged Core
Protons reside in the atom's nucleus and carry a positive electrical charge, equal in magnitude but opposite in sign to that of an electron. The number of protons in an atom's nucleus determines its atomic number and defines the element. For example, hydrogen has one proton, helium has two, and so on. Protons are significantly more massive than electrons.
2. Neutrons: The Neutral Nucleus Residents
Neutrons, also located in the nucleus, are electrically neutral, meaning they have no charge. They contribute to the atom's mass but not its charge. The number of neutrons in an atom can vary, leading to isotopes of the same element (atoms with the same number of protons but different numbers of neutrons).
3. Electrons: The Orbiting Negatively Charged Particles
Electrons are negatively charged particles that orbit the nucleus in regions called electron shells or orbitals. They are much smaller and lighter than protons and neutrons. The number of electrons in a neutral atom is equal to the number of protons. The arrangement of electrons in the electron shells determines the atom's chemical properties and how it interacts with other atoms.
Beyond the Triad: Quarks and Leptons
While protons, neutrons, and electrons were initially considered fundamental, further research revealed that protons and neutrons are themselves composed of even smaller particles called quarks.
Quarks: The Building Blocks of Protons and Neutrons
Quarks are elementary particles, meaning they are not currently believed to be made up of smaller constituents. There are six types, or "flavors," of quarks: up, down, charm, strange, top, and bottom. Each quark carries a fractional electric charge. Protons are made up of two up quarks and one down quark, while neutrons consist of one up quark and two down quarks.
The strong force, mediated by gluons, binds quarks together to form protons and neutrons. This force is incredibly strong at short distances, which is why quarks are always found bound within hadrons (particles composed of quarks). It's extremely difficult to isolate a single quark.
Leptons: The Electron's Family
Electrons belong to a class of particles called leptons. Leptons are fundamental particles that do not experience the strong force. Besides electrons, other leptons include muons and tau particles, along with their associated neutrinos. These particles play important roles in various processes within the atom and beyond.
The Standard Model of Particle Physics: A Comprehensive Framework
The Standard Model of particle physics is the most successful theory we have to describe the fundamental constituents of matter and their interactions. It encompasses quarks, leptons, and the force-carrying particles that mediate their interactions:
- Electromagnetic force: Mediated by photons.
- Weak nuclear force: Mediated by W and Z bosons. Responsible for radioactive decay.
- Strong nuclear force: Mediated by gluons. Responsible for holding quarks together.
- Gravitational force: While gravity is a fundamental force, it's not yet fully integrated into the Standard Model.
So, What Is the Smallest Part?
Given this information, we can revisit the initial question. The answer depends on the context:
- At the level of the atom's structure: Protons, neutrons, and electrons are the constituents.
- At the level of fundamental particles: Quarks and leptons (including electrons) are considered fundamental particles, not composed of anything smaller (as far as our current understanding goes).
Therefore, the smallest part of an atom, depending on your perspective, can be either the quarks and leptons that compose protons, neutrons, and electrons, or, more simply, the electrons, protons, and neutrons themselves.
Beyond the Standard Model: Open Questions and Future Research
Despite the success of the Standard Model, there are still unanswered questions and mysteries in particle physics:
- Dark matter and dark energy: These constitute the vast majority of the universe's mass-energy content, yet their nature remains unknown.
- Neutrino masses: The Standard Model predicts massless neutrinos, but experiments have shown they have tiny masses.
- The hierarchy problem: The huge discrepancy between the gravitational force and the other fundamental forces remains unexplained.
- Unification of forces: A grand unified theory (GUT) that unites the strong, weak, and electromagnetic forces is still a goal for physicists.
These open questions drive ongoing research, pushing the boundaries of our understanding of the universe's fundamental building blocks. The search for even more fundamental particles and forces continues, possibly revealing even smaller constituents of matter beyond what is currently known. New experiments and advancements in theoretical physics might one day lead to a revised understanding of what truly constitutes the smallest part of an atom, or even redefine what an "atom" itself signifies.
Conclusion: A Continuous Journey of Discovery
The journey to understanding the smallest part of an atom has been a remarkable adventure in scientific exploration. From the ancient philosophical concept of indivisible atoms to the complex Standard Model of particle physics, our knowledge has expanded tremendously. Yet, the quest continues. The discovery of quarks and leptons has provided significant progress, but the mysteries of dark matter, dark energy, and the unification of forces hint at a deeper layer of reality yet to be uncovered. The quest to understand the fundamental building blocks of the universe remains an ongoing and fascinating scientific pursuit. The "smallest part" is not a fixed point but rather a constantly evolving concept, reflecting our ever-increasing understanding of the intricacies of the cosmos.
Latest Posts
Latest Posts
-
Strong Electrolytes Weak Electrolytes And Nonelectrolytes
Mar 31, 2025
-
How Are Lipids And Carbohydrates Different
Mar 31, 2025
-
What Are Inclusions In A Cell
Mar 31, 2025
-
What Is The Basic Unit Of Measurement
Mar 31, 2025
-
Is It Possible To Love 2 People At Once
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Is The Smallest Part Of An Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
