What Makes Each Amino Acid Unique
Muz Play
Mar 24, 2025 · 6 min read
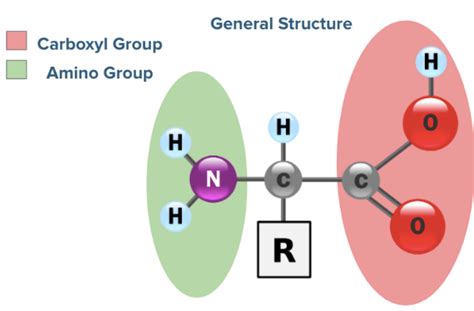
Table of Contents
What Makes Each Amino Acid Unique? A Deep Dive into the Building Blocks of Life
Amino acids. The very word conjures images of complex biochemistry, scientific jargon, and perhaps a slight feeling of intimidation. But these seemingly simple molecules are the fundamental building blocks of proteins, the workhorses of life. Understanding what makes each amino acid unique is crucial to grasping the incredible diversity and functionality of proteins, impacting everything from enzyme activity to immune response. This detailed exploration will delve into the structural features that differentiate the 20 standard amino acids, explaining how these subtle variations lead to a vast array of protein structures and functions.
The Core Structure: A Common Foundation
Before diving into the unique aspects, let's establish a baseline. All 20 standard amino acids share a common core structure:
- A central carbon atom (α-carbon): This carbon atom is bonded to four different groups.
- An amino group (-NH₂): This is a basic group, capable of accepting a proton (H⁺).
- A carboxyl group (-COOH): This is an acidic group, capable of donating a proton (H⁺).
- A hydrogen atom (-H): Simple, yet crucial to the overall structure.
- A side chain (R-group): This is the variable group, and it's the key to the unique properties of each amino acid.
It's the R-group, also known as the side chain, that dictates the amino acid's size, shape, charge, polarity, and reactivity. This seemingly small difference has enormous consequences for the protein's final three-dimensional structure and biological function.
Classifying Amino Acids Based on R-Group Properties
Amino acids are often categorized based on the properties of their R-groups. This classification helps us understand how these variations influence protein structure and function. The major categories include:
1. Nonpolar, Aliphatic Amino Acids
These amino acids have hydrophobic (water-fearing) side chains, meaning they tend to cluster together in the interior of proteins, away from the aqueous environment.
- Glycine (Gly, G): The simplest amino acid, with a single hydrogen atom as its R-group. Its small size allows for flexibility in protein folding. Its lack of a significant side chain makes it unique.
- Alanine (Ala, A): Has a methyl group (-CH₃) as its R-group. It's a relatively small and nonpolar amino acid.
- Valine (Val, V): Has a branched isopropyl group (-CH(CH₃)₂) as its R-group. Contributes to hydrophobic interactions.
- Leucine (Leu, L): Similar to valine, but with a longer isobutyl group (-CH₂CH(CH₃)₂). Another significant contributor to hydrophobic cores in proteins.
- Isoleucine (Ile, I): An isomer of leucine, with a different arrangement of the branched carbon atoms. Its unique structure influences protein packing.
- Methionine (Met, M): Contains a thioether (-SCH₃) group in its side chain. It's important for protein initiation during translation and has a slightly different hydrophobic character.
2. Aromatic Amino Acids
These amino acids possess aromatic rings in their side chains, contributing to their hydrophobic nature and ability to absorb UV light.
- Phenylalanine (Phe, F): Contains a benzene ring as its R-group. Its hydrophobic nature contributes to protein stability.
- Tyrosine (Tyr, Y): Similar to phenylalanine, but with a hydroxyl (-OH) group attached to the benzene ring. This hydroxyl group introduces a slight polarity, making it more reactive than phenylalanine.
- Tryptophan (Trp, W): Contains an indole ring in its side chain. This large, hydrophobic ring contributes significantly to protein structure and interacts with other molecules via its unique chemical properties.
3. Polar, Uncharged Amino Acids
These amino acids have polar side chains, meaning they are attracted to water molecules and often found on the surface of proteins, interacting with the surrounding environment.
- Serine (Ser, S): Contains a hydroxyl (-OH) group, making it polar and capable of forming hydrogen bonds. Often involved in enzymatic reactions.
- Threonine (Thr, T): Similar to serine, but with an additional methyl group. The presence of the hydroxyl group impacts its interactions within proteins.
- Cysteine (Cys, C): Contains a thiol (-SH) group, capable of forming disulfide bonds with other cysteine residues. These bonds are essential for stabilizing protein structures.
- Asparagine (Asn, N): Contains an amide group (-CONH₂), which allows for hydrogen bonding.
- Glutamine (Gln, Q): Similar to asparagine, but with an extra methylene group (-CH₂) in its side chain. This makes it less susceptible to certain types of reactions.
4. Positively Charged (Basic) Amino Acids
These amino acids have positively charged R-groups at physiological pH, making them highly reactive and often involved in protein-protein interactions.
- Lysine (Lys, K): Has a long, aliphatic chain ending in a positively charged amino group (-NH₃⁺). Often found on the surface of proteins, interacting with negatively charged molecules.
- Arginine (Arg, R): Contains a guanidinium group, which is strongly positively charged at physiological pH. It is critical for protein interactions and enzymatic activity.
- Histidine (His, H): Has an imidazole ring, which can be positively or neutrally charged depending on the pH. Its ability to act as both an acid and a base makes it essential in catalytic sites of many enzymes.
5. Negatively Charged (Acidic) Amino Acids
These amino acids have negatively charged R-groups at physiological pH due to the presence of carboxyl groups. They are also frequently involved in protein interactions.
- Aspartic Acid (Asp, D): Contains a carboxyl group (-COO⁻) in its side chain. Contributes to negative charges on the protein surface.
- Glutamic Acid (Glu, E): Similar to aspartic acid, but with an extra methylene group. Often involved in ionic interactions and protein binding.
The Impact of R-Group Variations on Protein Structure and Function
The unique properties of each amino acid's R-group directly influence the overall structure and function of the protein. These influences include:
- Protein Folding: Hydrophobic interactions between nonpolar side chains drive the folding of proteins into three-dimensional structures. Polar and charged side chains interact with the surrounding aqueous environment and participate in hydrogen bonding, contributing to the stability of the protein's folded state.
- Enzyme Activity: The precise arrangement of amino acids in the active site of an enzyme determines its substrate specificity and catalytic mechanism. Specific side chain interactions are crucial for substrate binding and catalysis.
- Protein-Protein Interactions: Charged and polar amino acid side chains play a crucial role in forming non-covalent interactions with other proteins, enabling the formation of protein complexes.
- Post-Translational Modifications: Some amino acids are susceptible to post-translational modifications (PTMs), altering their properties and affecting protein function. For example, serine, threonine, and tyrosine can be phosphorylated, influencing enzyme activity and signaling pathways. Cysteine residues can form disulfide bonds, enhancing protein stability.
Beyond the 20 Standard Amino Acids: A Wider World
While we've focused on the 20 standard amino acids, it's important to acknowledge the existence of other amino acids found in proteins, often resulting from post-translational modifications. These non-standard amino acids further enhance the diversity and functionality of proteins. Examples include hydroxyproline (in collagen), selenocysteine (an essential part of certain enzymes), and phosphoserine (a crucial component of many signaling pathways).
Conclusion: A Symphony of Subtle Differences
The remarkable diversity of proteins stems from the subtle yet significant differences in the R-groups of the 20 standard amino acids. The size, shape, charge, polarity, and reactivity of these side chains dictate how amino acids interact with each other and their environment, ultimately determining the protein's three-dimensional structure and its intricate biological function. Understanding these unique properties is crucial for advancing our knowledge of biology, medicine, and biotechnology, opening doors to the development of new therapeutics and technologies. The seemingly simple world of amino acids is, in reality, a complex and fascinating landscape of molecular interactions that underpin all life.
Latest Posts
Latest Posts
-
Solving Systems Of Equations By Substitution Answer Key
Mar 27, 2025
-
Person In The Environment Theory In Social Work
Mar 27, 2025
-
What Is The Difference Between Fractional And Simple Distillation
Mar 27, 2025
-
Is H30 An Acid Or Base
Mar 27, 2025
-
Identifying Acids And Bases By Their Chemical Formula
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about What Makes Each Amino Acid Unique . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
