What Particles Are Located In The Nucleus
Muz Play
Mar 18, 2025 · 6 min read
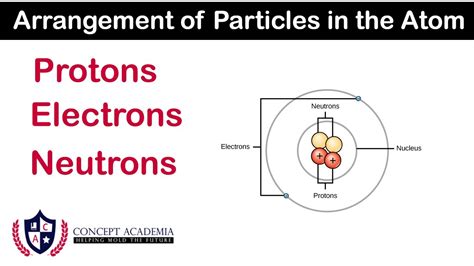
Table of Contents
What Particles are Located in the Nucleus? A Deep Dive into the Atomic Heart
The atom, the fundamental building block of matter, is a fascinating world of its own. While often depicted as a simple sphere, the atom possesses a complex internal structure. At the heart of this structure lies the nucleus, a tiny, dense region teeming with particles that determine the atom's properties and behavior. Understanding what particles are located in the nucleus is crucial to comprehending the nature of matter itself. This article delves deep into the nuclear landscape, exploring the key players and their interactions.
The Nucleus: A Dense and Powerful Core
The nucleus, despite its incredibly small size (about 1/100,000th the diameter of the atom), accounts for almost the entire mass of the atom. This density is a testament to the powerful forces at play within. It's here that we find two primary types of particles: protons and neutrons. These particles, collectively known as nucleons, are bound together by the strong nuclear force, an incredibly strong but short-range interaction that overcomes the electrostatic repulsion between the positively charged protons.
Protons: The Positively Charged Guardians
Protons carry a single positive electrical charge (+1). The number of protons in an atom's nucleus defines its atomic number, which uniquely identifies the element. For example, hydrogen has one proton (atomic number 1), helium has two (atomic number 2), and so on. The proton's charge is crucial for chemical reactions and the atom's overall behavior in electric and magnetic fields. Protons are also significantly more massive than electrons, adding substantially to the atom's overall mass.
Key characteristics of protons:
- Charge: +1
- Mass: Approximately 1.6726 × 10<sup>-27</sup> kg (approximately 1836 times the mass of an electron)
- Spin: ½ (fermion)
- Composition: Composed of three quarks (two up quarks and one down quark)
Neutrons: The Neutral Stabilizers
Neutrons, as their name suggests, carry no net electrical charge (0). Their presence in the nucleus is vital for stability. While protons repel each other due to their like charges, neutrons help to counteract this repulsion and hold the nucleus together through the strong nuclear force. The number of neutrons in a nucleus, along with the number of protons, determines the isotope of an element. Isotopes of the same element have the same number of protons but different numbers of neutrons. Some isotopes are stable, while others are radioactive and decay over time.
Key characteristics of neutrons:
- Charge: 0
- Mass: Slightly larger than a proton, approximately 1.6749 × 10<sup>-27</sup> kg
- Spin: ½ (fermion)
- Composition: Composed of three quarks (one up quark and two down quarks)
Beyond Protons and Neutrons: Exploring Subatomic Particles
The picture of the nucleus isn't complete without considering the subatomic particles that constitute protons and neutrons: quarks. These are fundamental particles, meaning they are not made up of smaller constituents. Protons and neutrons are each made up of three quarks bound together by the strong force mediated by gluons.
Quarks: The Fundamental Building Blocks
There are six types, or "flavors," of quarks: up, down, charm, strange, top, and bottom. Protons are composed of two up quarks and one down quark, while neutrons consist of one up quark and two down quarks. Each quark carries a fractional electric charge. Up quarks have a charge of +⅔, and down quarks have a charge of -⅓. The combination of these charges in protons and neutrons results in their overall charges of +1 and 0, respectively.
Key characteristics of quarks:
- Charge: Fractional charges (+⅔, -⅓)
- Mass: Vary considerably depending on the quark flavor
- Spin: ½ (fermion)
- Color Charge: Possess a property called "color charge," which interacts via the strong force.
Gluons: The Force Carriers of the Strong Interaction
The strong nuclear force, responsible for binding quarks together within protons and neutrons and holding nucleons together within the nucleus, is mediated by particles called gluons. Gluons are massless and carry the "color charge," allowing them to interact with quarks. The exchange of gluons between quarks is what leads to the strong force. The complexity of this interaction is described by Quantum Chromodynamics (QCD), a branch of physics that deals with the strong force and its effects.
Nuclear Stability and Radioactive Decay
The ratio of protons to neutrons in a nucleus plays a critical role in its stability. For lighter elements, a roughly equal number of protons and neutrons tends to lead to stable nuclei. However, as the atomic number increases, the number of neutrons required for stability exceeds the number of protons. This is because the strong nuclear force has a limited range, and the increasing electrostatic repulsion between protons requires more neutrons to provide enough strong force to hold the nucleus together.
When a nucleus is unstable, it undergoes radioactive decay, transforming into a more stable configuration. Several types of radioactive decay exist, including alpha decay (emission of an alpha particle, which is a helium nucleus), beta decay (emission of an electron or positron), and gamma decay (emission of a gamma ray). These decay processes often involve changes in the number of protons and neutrons, leading to the formation of different isotopes or elements.
The Nucleus and Nuclear Physics
The study of the nucleus and its constituents is the domain of nuclear physics. This field explores the properties and behavior of nuclei, including their stability, decay processes, and interactions with other particles. Nuclear physics has numerous applications, including:
- Nuclear energy: Utilizing nuclear fission (splitting of heavy nuclei) or fusion (combining light nuclei) to generate electricity.
- Nuclear medicine: Using radioactive isotopes for diagnosis and treatment of diseases.
- Material science: Modifying the properties of materials through irradiation or implantation of ions.
- Particle physics: Studying the fundamental particles and forces that govern the universe.
Exploring Further: Isotopes and Nuclear Models
The behavior of nuclei is further complicated by the existence of isotopes. Isotopes of an element have the same number of protons but different numbers of neutrons. This variation in neutron number impacts the stability and properties of the nucleus. Some isotopes are stable, while others are radioactive and decay over time, emitting radiation in the process. Understanding the different isotopes of an element is crucial in various fields, such as nuclear chemistry, nuclear medicine, and environmental science.
Various models attempt to explain the structure and behavior of nuclei. These models range from simple shell models that treat nucleons as independent particles moving in a potential well, to more complex models considering the interactions between nucleons and the effects of the strong nuclear force. The liquid drop model, for instance, views the nucleus as a drop of incompressible fluid, which helps explain some nuclear properties like binding energy. These models are crucial for predicting nuclear behavior and designing applications that harness nuclear energy and reactions.
Conclusion: The Nucleus – A Microcosm of the Universe
The nucleus, despite its tiny size, is a complex and fascinating structure that holds the key to understanding the fundamental properties of matter. The interplay between protons, neutrons, quarks, and gluons within the nucleus determines the atom's identity, stability, and interactions with its environment. Studying the nucleus unveils the secrets of the strong nuclear force, isotopes, and radioactive decay, leading to advancements in diverse fields ranging from nuclear energy to medicine and material science. The journey into the heart of the atom is a testament to the intricate and beautiful elegance of the universe's fundamental building blocks.
Latest Posts
Latest Posts
-
How Much Energy To Be At Zero Kinetic Energy
Mar 18, 2025
-
Current As A Function Of Time
Mar 18, 2025
-
Ode To Billy Joe Lyrics Meaning
Mar 18, 2025
-
Where Does The Light Independent Reaction Take Place
Mar 18, 2025
-
S P D F Blocks On The Periodic Table
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about What Particles Are Located In The Nucleus . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
