What Protonates An Amine At Ph 4
Muz Play
Mar 16, 2025 · 5 min read
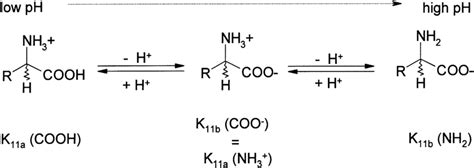
Table of Contents
What Protonates an Amine at pH 4? A Deep Dive into Acid-Base Chemistry
Amine protonation, a fundamental process in chemistry and biology, is heavily influenced by the surrounding pH. Understanding what protonates an amine at a specific pH, such as pH 4, requires a detailed understanding of acid-base equilibria and the properties of amines themselves. This article will explore this topic comprehensively, examining the factors that contribute to amine protonation at pH 4 and the implications of this process.
Understanding Amines and Their Properties
Amines are organic compounds derived from ammonia (NH₃) by replacing one or more hydrogen atoms with alkyl or aryl groups. They are characterized by the presence of a nitrogen atom with a lone pair of electrons, which is crucial for their basic properties. This lone pair readily accepts a proton (H⁺), leading to the formation of an ammonium ion (R₃NH⁺). The strength of an amine as a base, its propensity to accept a proton, depends on several factors:
Factors Affecting Amine Basicity:
-
Alkyl Substitution: Alkyl groups are electron-donating, increasing the electron density on the nitrogen atom and making the amine more basic. Tertiary amines (R₃N) are generally more basic than secondary (R₂NH) and primary (RNH₂) amines.
-
Aryl Substitution: Aryl groups are electron-withdrawing, decreasing the electron density on the nitrogen atom and reducing the basicity of the amine. Aromatic amines (anilines) are significantly weaker bases than aliphatic amines.
-
Resonance Effects: If the nitrogen atom is part of a conjugated system, resonance can delocalize the lone pair, reducing its availability for protonation and decreasing basicity.
-
Steric Hindrance: Bulky substituents around the nitrogen atom can hinder the approach of a proton, reducing the rate of protonation but not necessarily the equilibrium constant.
The Role of pH in Amine Protonation
The pH of a solution dictates the concentration of hydronium ions (H₃O⁺), which are the primary proton donors in aqueous solutions. A lower pH signifies a higher concentration of H₃O⁺, increasing the likelihood of protonation.
At pH 4, the solution is acidic. This means the concentration of H₃O⁺ is significantly higher than the concentration of hydroxide ions (OH⁻). This high concentration of hydronium ions is the primary factor driving amine protonation at this pH.
What Specifically Protonates the Amine at pH 4?
The answer is straightforward: hydronium ions (H₃O⁺). In an aqueous solution at pH 4, hydronium ions are abundant. These ions act as Brønsted-Lowry acids, donating a proton to the amine's lone pair of electrons. The reaction can be represented as follows:
R₃N + H₃O⁺ ⇌ R₃NH⁺ + H₂O
This reaction is an equilibrium, meaning that both the protonated and unprotonated forms of the amine coexist in solution. The position of the equilibrium (the ratio of protonated to unprotonated amine) depends on the pKa of the amine and the pH of the solution.
pKa and the Amine Protonation Equilibrium
The pKa of an amine is a measure of its acidity, specifically, the tendency to donate a proton. However, in this context, we're considering its conjugate acid, the ammonium ion (R₃NH⁺), and its tendency to donate a proton back to the solution. A lower pKa indicates a stronger acid, meaning the ammonium ion more readily loses a proton. The relationship between pKa, pH, and the degree of protonation is given by the Henderson-Hasselbalch equation:
pH = pKa + log ([R₃N]/[R₃NH⁺])
Where:
- pH is the pH of the solution
- pKa is the pKa of the ammonium ion (conjugate acid of the amine)
- [R₃N] is the concentration of the unprotonated amine
- [R₃NH⁺] is the concentration of the protonated amine
At pH = pKa, the concentrations of the protonated and unprotonated forms are equal, meaning that 50% of the amine is protonated. If the pH is lower than the pKa, the concentration of the protonated form ([R₃NH⁺]) will be higher, and vice-versa.
Predicting Amine Protonation at pH 4: A Practical Example
Let's consider a specific example. Suppose we have an aliphatic amine with a pKa of 10.5 (a relatively common range for aliphatic amines). At pH 4, which is significantly lower than the pKa, the Henderson-Hasselbalch equation predicts that a substantial fraction of the amine will be protonated.
pH = pKa + log ([R₃N]/[R₃NH⁺]) 4 = 10.5 + log ([R₃N]/[R₃NH⁺]) log ([R₃N]/[R₃NH⁺]) = -6.5 [R₃N]/[R₃NH⁺] = 10⁻⁶⋅⁵ ≈ 3.2 x 10⁻⁷
This indicates that the concentration of the protonated amine ([R₃NH⁺]) is vastly greater than the concentration of the unprotonated amine ([R₃N]). Therefore, at pH 4, this amine will be almost entirely protonated.
Influence of Other Factors
While hydronium ions are the primary protonating species at pH 4, other weaker acids present in the solution can contribute to amine protonation, albeit to a much lesser extent. The significance of these contributions is negligible compared to the impact of the abundant hydronium ions at pH 4.
Implications of Amine Protonation at pH 4
The protonation of amines at pH 4 has several important implications:
-
Solubility: Protonated amines are often more soluble in aqueous solutions than their unprotonated counterparts due to the increased polarity resulting from the positive charge.
-
Reactivity: The protonated form of the amine exhibits different reactivity compared to the unprotonated form. For instance, the protonated form is less nucleophilic.
-
Biological Significance: Amine protonation plays a crucial role in various biological processes, including enzyme catalysis, protein structure, and drug-receptor interactions. Many biological systems operate at near-neutral to slightly acidic pH values (pH 4-7) where amine protonation is relevant.
-
Analytical Chemistry: The ability to control the extent of amine protonation through pH manipulation is important in analytical techniques such as separation and quantification of amines by chromatography.
Conclusion
At pH 4, the abundant hydronium ions (H₃O⁺) are the primary species responsible for the protonation of amines. The extent of protonation depends on the pKa of the specific amine and the pH of the solution, governed by the Henderson-Hasselbalch equation. This process significantly impacts the amine's solubility, reactivity, and biological function. Understanding the factors influencing amine protonation is crucial in various scientific fields, ranging from organic chemistry to biochemistry and analytical chemistry. While other weak acids in solution can theoretically contribute, their impact at pH 4 is overshadowed by the high concentration of hydronium ions. Therefore, in practical terms, at pH 4, it is the hydronium ion that is the key player in amine protonation.
Latest Posts
Latest Posts
-
Whats The Derivative Of A Constant
Mar 17, 2025
-
Differential Rate Law For Zero Order Reaction
Mar 17, 2025
-
Cell The Basic Unit Of Life
Mar 17, 2025
-
An Increase In The Aggregate Expenditures Schedule
Mar 17, 2025
-
A Temporary Mixture The Particles Will Eventually Settle
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Protonates An Amine At Ph 4 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
