What Subatomic Particles Make Up An Atom
Muz Play
Mar 26, 2025 · 5 min read
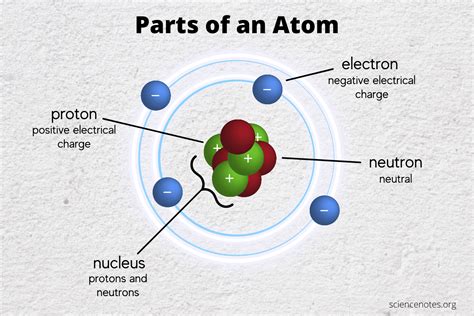
Table of Contents
What Subatomic Particles Make Up an Atom? A Deep Dive into the Building Blocks of Matter
The atom, once considered the smallest indivisible unit of matter, is now understood to be a complex system composed of even smaller particles called subatomic particles. Understanding these fundamental building blocks is crucial to grasping the nature of matter and the forces that govern the universe. This article delves deep into the subatomic world, exploring the particles that constitute atoms and their roles in atomic structure and behavior.
The Core Components: Protons, Neutrons, and Electrons
The three primary subatomic particles that make up an atom are protons, neutrons, and electrons. These particles possess distinct properties that define their behavior and their contribution to the atom's overall characteristics.
Protons: The Positive Charge Carriers
Protons reside within the atom's nucleus, a dense central region. Each proton carries a single positive electrical charge (+1). The number of protons in an atom's nucleus defines its atomic number and uniquely identifies the element. For example, hydrogen (H) has one proton, helium (He) has two, and carbon (C) has six. This fundamental characteristic dictates the atom's chemical properties and its place on the periodic table. Protons are significantly more massive than electrons, contributing substantially to the atom's overall mass.
Neutrons: The Neutral Partners
Neutrons, like protons, are located within the atom's nucleus. As their name suggests, they carry no electrical charge (0). Their primary role is to contribute to the nucleus's mass and stability. The number of neutrons in an atom's nucleus can vary, even within the same element, giving rise to isotopes. Isotopes are atoms of the same element with the same number of protons but different numbers of neutrons. Some isotopes are stable, while others are radioactive, decaying over time. The mass number of an atom is the sum of its protons and neutrons.
Electrons: The Orbiting Negatives
Unlike protons and neutrons, electrons are found outside the nucleus in regions called electron shells or energy levels. These shells are not physical orbits, but rather regions of probability where electrons are most likely to be found. Electrons carry a single negative electrical charge (-1), balancing the positive charge of the protons in a neutral atom. The number of electrons in an atom is typically equal to the number of protons, ensuring overall electrical neutrality. Electrons play a crucial role in chemical bonding and reactions, as their interactions with other atoms determine the formation of molecules and compounds. The arrangement of electrons in energy levels significantly influences the atom's chemical reactivity.
Diving Deeper: The Quark Structure of Protons and Neutrons
While protons, neutrons, and electrons were once considered fundamental particles, further research revealed that protons and neutrons are themselves composed of even smaller particles called quarks.
Quarks: The Fundamental Building Blocks
Quarks are elementary particles that interact through the strong force, responsible for binding protons and neutrons together within the nucleus. There are six types, or "flavors," of quarks: up, down, charm, strange, top, and bottom. Each quark possesses a fractional electric charge, unlike the integer charges of protons and electrons.
- Up quarks have a charge of +2/3.
- Down quarks have a charge of -1/3.
Protons and neutrons are each composed of three quarks:
- Proton: Two up quarks and one down quark (2/3 + 2/3 - 1/3 = +1)
- Neutron: One up quark and two down quarks (2/3 - 1/3 - 1/3 = 0)
The strong force, mediated by gluons, holds these quarks together within protons and neutrons. Gluons are massless particles that carry the strong nuclear force. The complexity of the strong force and the interactions between quarks are described by Quantum Chromodynamics (QCD), a fundamental theory in particle physics.
Beyond the Basics: Other Subatomic Particles
While protons, neutrons, and electrons are the primary components of atoms, many other subatomic particles exist, often playing roles in various nuclear processes and particle interactions. These include:
-
Leptons: Electrons are part of a larger family of particles called leptons, which also includes muons, tau particles, and their associated neutrinos. Leptons are fundamental particles that do not experience the strong force.
-
Bosons: These particles mediate fundamental forces. Beyond gluons (which mediate the strong force), we have:
- Photons: Mediate the electromagnetic force.
- W and Z bosons: Mediate the weak force, responsible for radioactive decay.
- Higgs boson: Associated with the Higgs field, which gives particles mass.
-
Antiparticles: For every particle, there exists an antiparticle with the same mass but opposite charge and other quantum numbers. When a particle and its antiparticle collide, they annihilate each other, releasing energy.
Atomic Structure and Stability
The arrangement of protons, neutrons, and electrons within an atom dictates its stability and reactivity. The strong nuclear force holds the protons and neutrons together in the nucleus, overcoming the electromagnetic repulsion between the positively charged protons. The number of neutrons relative to protons influences nuclear stability. Atoms with an unstable nucleus may undergo radioactive decay, transforming into a more stable configuration.
The electron shells surrounding the nucleus determine the atom's chemical behavior. Electrons in the outermost shell, known as valence electrons, are involved in chemical bonding with other atoms. Atoms tend to react with each other to achieve a stable electron configuration, often by filling their outermost shell with electrons. This drive for stability underlies the formation of molecules and compounds.
Applications and Further Exploration
The understanding of subatomic particles has revolutionized various fields, including:
- Nuclear energy: Harnessing the energy released during nuclear reactions, which involve changes in the atomic nucleus.
- Medical imaging and treatment: Techniques like PET scans and radiation therapy rely on understanding the behavior of subatomic particles.
- Materials science: Designing new materials with specific properties by manipulating atomic structure.
- Particle physics: Continuously exploring the fundamental constituents of matter and the forces that govern them.
The journey into the subatomic world is far from over. Ongoing research continues to uncover new insights into the nature of matter, exploring the mysteries of dark matter, dark energy, and the fundamental forces of the universe. The study of subatomic particles is crucial for advancing our understanding of the cosmos and developing new technologies. The basic building blocks we've explored – protons, neutrons, electrons, and their constituent quarks – represent a significant step in this ongoing scientific quest. Further exploration of the Standard Model of particle physics and beyond promises to reveal even more profound secrets about the universe and its composition.
Latest Posts
Latest Posts
-
What Are The Characteristics Of Covalent Compounds
Mar 29, 2025
-
What Is Found In Animal Cells But Not Plant Cells
Mar 29, 2025
-
Surface Integral Of A Sphere In Spherical Coordinates
Mar 29, 2025
-
Report Sheet Chemical Reactions Experiment 4
Mar 29, 2025
-
What Makes A Good Recrystallization Solvent
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about What Subatomic Particles Make Up An Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
