What Are The Characteristics Of Covalent Compounds
Muz Play
Mar 29, 2025 · 7 min read
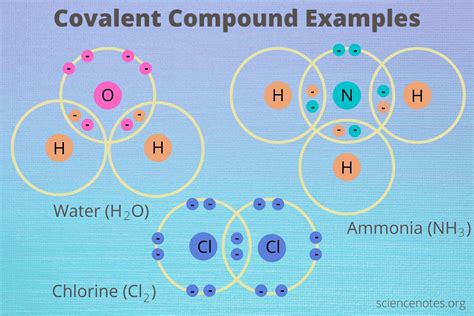
Table of Contents
What Are the Characteristics of Covalent Compounds?
Covalent compounds, also known as molecular compounds, represent a fundamental class of chemical substances formed through the sharing of electrons between atoms. This sharing, as opposed to the electron transfer in ionic compounds, results in a unique set of properties that distinguish them from other compound types. Understanding these characteristics is crucial for predicting the behavior of covalent compounds in various contexts, from their physical states to their reactivity. This comprehensive guide will delve into the key characteristics of covalent compounds, exploring their structure, bonding, physical properties, and chemical behavior.
Understanding Covalent Bonding
At the heart of covalent compounds lies the covalent bond, a chemical link formed when two atoms share one or more pairs of electrons. This sharing allows each atom to achieve a more stable electron configuration, often resembling the noble gas configuration with a full outer electron shell. The shared electrons are attracted to the nuclei of both atoms, holding them together in a molecule.
Types of Covalent Bonds:
- Single Covalent Bond: Involves the sharing of one electron pair between two atoms (e.g., H₂).
- Double Covalent Bond: Involves the sharing of two electron pairs between two atoms (e.g., O₂).
- Triple Covalent Bond: Involves the sharing of three electron pairs between two atoms (e.g., N₂). Triple bonds are stronger and shorter than double bonds, and double bonds are stronger and shorter than single bonds.
The strength of the covalent bond depends on several factors, including the number of shared electron pairs and the electronegativity difference between the atoms involved. A larger electronegativity difference can lead to a polar covalent bond, where the shared electrons are pulled more towards one atom than the other. We'll explore this further in the next section.
Properties of Covalent Compounds
The characteristics of covalent compounds are directly related to the nature of their covalent bonds and the resulting molecular structure. These properties are broadly categorized into physical and chemical characteristics.
Physical Properties:
-
Low Melting and Boiling Points: Covalent compounds generally have relatively low melting and boiling points compared to ionic compounds. This is because the intermolecular forces (forces of attraction between molecules) are relatively weak. These forces, such as van der Waals forces, hydrogen bonds, and dipole-dipole interactions, are much weaker than the strong electrostatic forces found in ionic compounds. Therefore, less energy is required to overcome these weaker forces, resulting in lower melting and boiling points.
-
Poor Electrical Conductivity: Covalent compounds typically do not conduct electricity in either their solid or liquid states. This is because they do not contain freely moving charged particles (ions or electrons) as found in ionic compounds or metals. The electrons are tightly bound within the covalent bonds and are not free to move and carry an electric current.
-
Solubility: The solubility of covalent compounds varies greatly depending on the polarity of the molecule and the polarity of the solvent. Polar covalent compounds tend to dissolve in polar solvents (like water), while nonpolar covalent compounds tend to dissolve in nonpolar solvents (like organic solvents). "Like dissolves like" is a useful rule of thumb here.
-
States of Matter at Room Temperature: Covalent compounds can exist in all three states of matter at room temperature. Gases (e.g., CO₂, O₂), liquids (e.g., H₂O, ethanol), and solids (e.g., sugar, diamond) are all examples of covalent compounds. The state of matter depends largely on the strength of the intermolecular forces and the molecular weight of the compound.
-
Low Hardness and Brittleness: Covalent solids are generally softer and more brittle than ionic solids. This is because the covalent bonds are directional, meaning they exist between specific atoms, and lack the strong, three-dimensional network of ionic bonds. When stress is applied, the directional bonds are easily broken, resulting in brittle fracture. However, network covalent solids, such as diamond, are exceptions to this rule, possessing exceptional hardness due to their strong, extensive network of covalent bonds.
Chemical Properties:
-
Reactivity: The reactivity of covalent compounds is highly variable and depends on many factors, including the specific atoms involved, the presence of multiple bonds, and the presence of functional groups. Some covalent compounds are very reactive (e.g., chlorine gas), while others are quite unreactive (e.g., methane).
-
Combustion: Many covalent compounds, particularly those containing carbon and hydrogen, are combustible, reacting readily with oxygen to produce carbon dioxide, water, and heat.
-
Hydrolysis: Some covalent compounds react with water (hydrolyze) to form different products. This often occurs with compounds containing highly polar bonds, such as esters and amides.
-
Reactions with Acids and Bases: The reaction of covalent compounds with acids and bases is also varied. Some are resistant to attack by acids and bases, while others may undergo reactions such as substitution or addition reactions.
Examples of Covalent Compounds and Their Applications
Covalent compounds are ubiquitous in nature and have a wide range of applications in various industries. Here are a few examples:
- Water (H₂O): Essential for life, used as a solvent, coolant, and reactant in countless processes.
- Carbon Dioxide (CO₂): Important greenhouse gas, also used in carbonated drinks and fire extinguishers.
- Glucose (C₆H₁₂O₆): A simple sugar, crucial energy source for living organisms.
- Methane (CH₄): A primary component of natural gas, used as a fuel source.
- Ethanol (C₂H₅OH): Used as a solvent, fuel additive, and in alcoholic beverages.
- Silicon Dioxide (SiO₂): Main component of sand and quartz, used in glass manufacturing and electronics.
- Polymers: Large molecules composed of repeating units, used in plastics, fabrics, and other materials. Examples include polyethylene (plastic bags), nylon (fabrics), and silicone (sealants).
- Diamonds: A form of pure carbon with a giant covalent structure, prized for its hardness and brilliance.
- Graphite: Another form of pure carbon with a layered structure, used in pencils, lubricants, and electrodes.
These examples highlight the diverse range of applications of covalent compounds. Their properties, ranging from hardness to reactivity, dictate their use in various technologies and everyday products.
Distinguishing Covalent Compounds from Ionic Compounds
While both covalent and ionic compounds are composed of atoms, their bonding mechanisms and resulting properties differ significantly. This distinction is crucial for understanding their behavior. Here's a comparison:
| Feature | Covalent Compounds | Ionic Compounds |
|---|---|---|
| Bonding | Sharing of electrons | Transfer of electrons |
| Melting Point | Generally low | Generally high |
| Boiling Point | Generally low | Generally high |
| Electrical Conductivity | Poor, generally non-conductive | Good when molten or dissolved in solution |
| Solubility | Varies depending on polarity; like dissolves like | Often soluble in water |
| Hardness | Generally soft and brittle (exceptions exist) | Generally hard and brittle |
| Examples | Water, methane, glucose, polymers | Sodium chloride (table salt), magnesium oxide |
Understanding these differences helps in predicting the properties and behaviors of different chemical substances.
Advanced Concepts in Covalent Bonding
Beyond the basic concepts, several advanced aspects of covalent bonding enhance our comprehension of covalent compounds:
-
Resonance: In some molecules, the bonding electrons cannot be described by a single Lewis structure. Resonance structures are used to represent the delocalized electrons, resulting in an average bond order that strengthens the molecule. Benzene is a classic example of resonance.
-
Delocalization: In conjugated systems, electrons are spread over multiple atoms, rather than being localized between two specific atoms. This delocalization contributes to stability and specific chemical reactivity.
-
Hybridization: Atomic orbitals can combine to form hybrid orbitals, which allow for optimal overlap and bonding in molecules. This concept explains the geometries of many covalent molecules.
-
Polarity and Dipole Moments: The difference in electronegativity between atoms in a covalent bond can create a dipole moment, making the molecule polar. This polarity significantly impacts the physical and chemical properties of the compound, affecting solubility and intermolecular forces.
-
Intermolecular Forces: While covalent bonds hold atoms within a molecule together, intermolecular forces attract different molecules to each other. These forces, including van der Waals forces, dipole-dipole interactions, and hydrogen bonding, influence many physical properties such as melting and boiling points and solubility. Understanding these forces is crucial for predicting the behavior of molecules in various states and environments.
Conclusion
Covalent compounds form the backbone of a vast array of materials found in nature and utilized in countless applications. Their unique characteristics, stemming from the sharing of electrons between atoms, dictate their behavior and make them indispensable in various technological, biological, and industrial processes. By understanding the intricacies of covalent bonding, the nature of intermolecular forces, and the properties that arise from these interactions, we gain a deeper appreciation for the diverse world of covalent compounds and their significant roles in our lives. Further exploration into the advanced concepts discussed will provide an even more thorough understanding of these fascinating chemical species.
Latest Posts
Latest Posts
-
Principle Of Conservation Of Angular Momentum
Mar 31, 2025
-
Thesis Statement Of A Narrative Essay
Mar 31, 2025
-
Ionic Compounds Dissociate In Water Into
Mar 31, 2025
-
The Ends Of Long Bones Are Called The
Mar 31, 2025
-
Is Ph A Chemical Or Physical Property
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Are The Characteristics Of Covalent Compounds . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
