Is Ph A Chemical Or Physical Property
Muz Play
Mar 31, 2025 · 5 min read
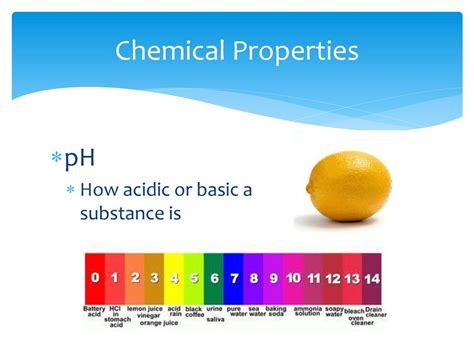
Table of Contents
Is pH a Chemical or Physical Property? A Deep Dive
The question of whether pH is a chemical or physical property often sparks debate. While seemingly straightforward, the answer requires a nuanced understanding of both pH itself and the fundamental distinctions between chemical and physical properties. This article delves into the intricacies of pH, exploring its nature, measurement, and implications to definitively answer this question and clarify any lingering confusion.
Understanding pH: A Measure of Acidity and Alkalinity
pH, a term derived from the potential of Hydrogen, quantifies the acidity or alkalinity of a solution. It represents the negative logarithm (base 10) of the hydrogen ion (H⁺) concentration. A lower pH value (below 7) indicates a higher concentration of H⁺ ions, signifying an acidic solution. Conversely, a higher pH value (above 7) denotes a lower concentration of H⁺ ions and a more alkaline (basic) solution. A pH of 7 signifies neutrality, representing an equal concentration of H⁺ and hydroxide (OH⁻) ions.
Key Characteristics of pH:
- Scale: The pH scale ranges from 0 to 14, with 7 representing neutrality.
- Logarithmic: Each whole number change on the pH scale represents a tenfold change in H⁺ ion concentration. For example, a solution with a pH of 3 is ten times more acidic than a solution with a pH of 4.
- Indicator Dependent: pH measurement relies on indicators that change color depending on the H⁺ ion concentration. These indicators form the basis of visual pH testing, while electronic pH meters offer precise digital readings.
- Context Dependent: The pH of a solution can vary greatly depending on factors like temperature, pressure, and the presence of other substances.
The Distinction Between Chemical and Physical Properties
Before classifying pH, it's crucial to establish a clear distinction between chemical and physical properties.
Physical Properties: These describe the characteristics of a substance that can be observed or measured without changing its chemical composition. Examples include color, density, melting point, boiling point, and solubility. These properties can be altered without fundamentally changing the substance's molecular structure.
Chemical Properties: These describe a substance's ability to undergo a chemical change or reaction, resulting in the formation of a new substance with different chemical properties. Examples include flammability, reactivity with acids or bases, and oxidation state. These properties are only observable when the substance undergoes a chemical transformation.
The Case for pH as a Chemical Property
The argument for classifying pH as a chemical property hinges on its direct relationship to chemical reactions and the behavior of substances in solutions. Several key aspects support this classification:
1. pH's Influence on Chemical Reactions:
pH significantly impacts the rate and direction of numerous chemical reactions. Many reactions proceed optimally within a specific pH range, while extreme pH values can inhibit or even prevent reactions altogether. Enzyme activity, a cornerstone of biological processes, is highly pH-dependent. Changes in pH can alter the enzyme's structure, impacting its catalytic function.
2. pH as a Measure of Chemical Equilibrium:
pH reflects the equilibrium between H⁺ and OH⁻ ions in a solution. This equilibrium is governed by chemical reactions, specifically the dissociation of water molecules into H⁺ and OH⁻ ions. Measuring pH therefore provides insight into the underlying chemical equilibrium within the solution.
3. pH-Dependent Chemical Reactions:
Many chemical reactions are directly influenced by pH. For instance, acid-base reactions are fundamentally defined by the transfer of protons (H⁺ ions), directly related to pH. The pH of a solution dictates whether a particular acid-base reaction will favor product formation or reactant formation. Furthermore, numerous precipitation reactions are pH-dependent, where changes in pH can cause the formation or dissolution of precipitates.
4. pH and Chemical Reactivity:
The pH of a substance dictates its reactivity towards other substances. A highly acidic solution (low pH) is significantly more reactive than a neutral or alkaline solution, readily participating in reactions with metals, bases, and certain organic compounds. This difference in reactivity is inherently a chemical property.
Counterarguments and Nuances
While the chemical property argument holds strong, some counterarguments suggest a physical property perspective. These arguments often focus on the methods of pH measurement:
-
Physical Measurement Methods: pH meters, though measuring a chemical property, rely on physical principles—electrical potential difference—for their measurements. This seemingly physical measurement aspect could lead to the misinterpretation of pH as a physical property. However, the underlying phenomenon being measured remains chemical.
-
Observable Effects: The color changes observed in pH indicators are physical changes. However, this change is a consequence of a chemical interaction between the indicator and the H⁺ ions, not the property itself.
Conclusion: pH – Predominantly a Chemical Property
Despite the nuances in measurement and observation, the preponderance of evidence strongly suggests that pH is primarily a chemical property. Its direct influence on chemical reactions, its representation of chemical equilibrium, and its impact on the reactivity of substances firmly place it within the realm of chemical properties. The physical methods used to measure pH do not negate its inherent chemical nature.
Further Exploration: Applications of pH Understanding
The understanding of pH is crucial across numerous scientific disciplines and practical applications:
- Chemistry: Acid-base titrations, buffer solutions, and various reaction optimization procedures heavily rely on pH control.
- Biology: pH regulation is essential for maintaining the functionality of biological systems, including enzymatic reactions, cellular processes, and organismal homeostasis.
- Environmental Science: pH measurements are vital in assessing water quality, soil health, and atmospheric conditions. Acid rain, for example, is characterized by a lowered pH, significantly impacting ecosystems.
- Medicine: pH plays a critical role in bodily functions and the efficacy of medications. Maintaining proper blood pH is vital for health, and deviations can lead to serious medical complications.
- Industry: Many industrial processes, including food processing, wastewater treatment, and chemical manufacturing, require precise pH control for optimal efficiency and product quality.
Understanding the chemical nature of pH is not just an academic exercise; it's fundamentally important in countless practical applications and scientific fields. This knowledge is crucial for addressing environmental issues, advancing medical treatments, and optimizing industrial processes. As our understanding of chemical reactions and their impact on the world around us continues to deepen, the importance of pH and its chemical nature will remain paramount.
Latest Posts
Latest Posts
-
How Do You Find The Boiling Point Of A Solution
Apr 02, 2025
-
What Happens To Ionization Energy Down A Group
Apr 02, 2025
-
How Many Grams In A Molecule
Apr 02, 2025
-
Is Cl A Metal Or Nonmetal
Apr 02, 2025
-
Microscopic Anatomy And Organization Of Skeletal Muscle
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Is Ph A Chemical Or Physical Property . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
