Ionic Compounds Dissociate In Water Into
Muz Play
Mar 31, 2025 · 6 min read
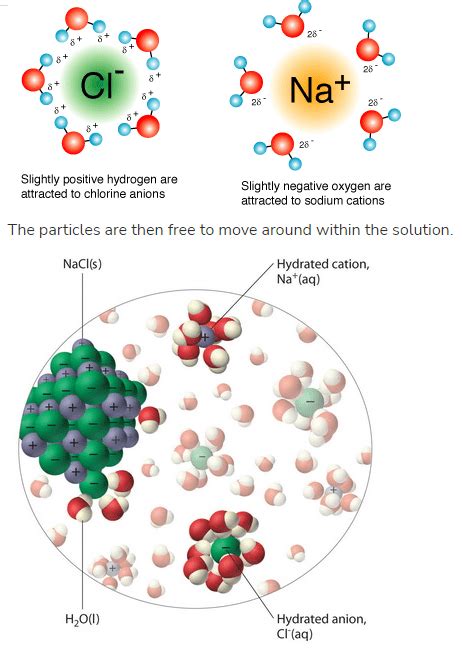
Table of Contents
Ionic Compounds Dissociate in Water Into: A Deep Dive into Dissolution and its Implications
Ionic compounds, characterized by the electrostatic attraction between oppositely charged ions, exhibit a unique behavior when introduced to water: they dissociate. This process, fundamental to many chemical and biological processes, involves the separation of ions from the crystalline lattice structure and their subsequent dispersion into the aqueous solution. Understanding why and how this dissociation occurs is crucial to grasping a wide range of concepts in chemistry. This article will delve into the intricacies of ionic compound dissociation in water, exploring the underlying mechanisms, influencing factors, and significant implications.
The Nature of Ionic Bonds and Water Molecules
Before exploring the dissociation process, let's establish a foundation by understanding the nature of ionic bonds and the unique properties of water.
Ionic Bonds: The Electrostatic Glue
Ionic bonds are formed through the electrostatic attraction between positively charged ions (cations) and negatively charged ions (anions). This attraction arises from the transfer of electrons from one atom to another, resulting in a net positive charge on the atom that loses electrons and a net negative charge on the atom that gains electrons. The strength of the ionic bond depends on several factors, including the charges of the ions and the distance between them. Larger charges and smaller distances lead to stronger bonds. These bonds hold the ions together in a highly ordered, crystalline structure.
Water: The Universal Solvent
Water (H₂O) possesses exceptional properties that make it an excellent solvent for many ionic compounds. Its polar nature is paramount. The oxygen atom in a water molecule is more electronegative than the hydrogen atoms, resulting in a partial negative charge (δ-) on the oxygen and partial positive charges (δ+) on the hydrogens. This polarity creates a dipole moment, making water a polar molecule. This polarity allows water molecules to effectively interact with and surround ions, facilitating their separation from the crystal lattice.
The Dissociation Process: A Step-by-Step Look
The dissociation of an ionic compound in water is a multi-step process that can be described as follows:
1. Hydration of Ions
As water molecules approach the ionic crystal, their polar nature plays a crucial role. The partially negative oxygen atoms of water molecules are attracted to the positive cations in the crystal lattice, while the partially positive hydrogen atoms are attracted to the negative anions. This attraction weakens the electrostatic forces holding the ions together in the crystal. This process is known as hydration.
2. Breaking the Ionic Bonds
The hydrated water molecules effectively shield the ions from each other, reducing the electrostatic attraction between them. Eventually, the attractive forces between the ions and the water molecules overcome the ionic bonds holding the crystal lattice together. This leads to the breaking of ionic bonds, causing the individual ions to separate from the crystal structure.
3. Dispersion of Ions
Once separated, the ions become surrounded by a layer of water molecules, a process known as solvation. This solvation shell stabilizes the ions in solution, preventing them from re-combining. The ions are now free to move independently within the solution. The degree of this movement depends on factors such as temperature and concentration.
Factors Influencing the Extent of Dissociation
Several factors can influence the extent to which an ionic compound dissociates in water:
1. Lattice Energy
The lattice energy is a measure of the strength of the electrostatic forces holding the ions together in the crystal lattice. A high lattice energy indicates strong bonds, making it more difficult for water molecules to separate the ions. Compounds with high lattice energies tend to be less soluble and therefore exhibit less dissociation.
2. Hydration Energy
Hydration energy is the energy released when water molecules surround and interact with ions. A high hydration energy means that the interaction between the ions and water molecules is strong, favoring dissociation. Compounds with high hydration energies tend to be more soluble and therefore exhibit greater dissociation.
3. Ion Size and Charge
The size and charge of the ions significantly impact both lattice energy and hydration energy. Smaller ions with higher charges have stronger electrostatic attractions (higher lattice energy) and stronger interactions with water molecules (higher hydration energy). The balance between these two factors determines the extent of dissociation. Generally, smaller, highly charged ions tend to show greater hydration and therefore greater dissociation.
4. Temperature
Increasing the temperature increases the kinetic energy of the water molecules, leading to more frequent and forceful collisions with the ionic crystal. This enhanced collision frequency can aid in overcoming the lattice energy and facilitate dissociation. Hence, solubility and dissociation typically increase with temperature.
5. Concentration
The concentration of the ionic compound in the solution also plays a role. At low concentrations, the dissociation is typically more complete. However, as the concentration increases, the likelihood of ion pairs reforming increases, leading to a decrease in the extent of dissociation.
Consequences of Dissociation: Conductivity and Reactivity
The dissociation of ionic compounds in water has significant consequences, profoundly impacting the properties and reactivity of the resulting solution:
Electrical Conductivity
One of the most striking consequences of dissociation is the enhanced electrical conductivity of the solution. Free-moving ions in the solution can carry an electric current, making the solution electrically conductive. This property is widely utilized in various applications, including electroplating, batteries, and electrochemical sensors.
Chemical Reactivity
Dissociation significantly impacts the chemical reactivity of the dissolved ionic compound. The separated ions can participate in various chemical reactions, significantly increasing their reactivity compared to the undissociated solid. This increased reactivity underlies many crucial chemical and biological processes.
Osmotic Pressure
Dissociated ions contribute to the osmotic pressure of a solution. Osmotic pressure is the pressure required to prevent the flow of water across a semipermeable membrane separating two solutions of different concentrations. This property plays a crucial role in biological systems, affecting processes such as water transport across cell membranes.
Applications and Examples
The understanding and application of ionic compound dissociation are ubiquitous across various fields:
-
Biological Systems: Dissociation is crucial for many biological processes, including nerve impulse transmission, muscle contraction, and enzyme activity. Many biological molecules are ionic in nature and rely on dissociation for their proper function.
-
Medicine: Many pharmaceuticals are ionic compounds that rely on dissociation to achieve their therapeutic effects. The solubility and bioavailability of these drugs are directly related to their dissociation behavior.
-
Industrial Processes: Electroplating, the production of metals, and various electrochemical processes rely heavily on the conductivity of solutions created by dissociated ions.
-
Environmental Science: Understanding the dissociation of ionic compounds is crucial for evaluating water quality, managing pollution, and predicting the fate of pollutants in the environment.
Conclusion: A Fundamental Process with Far-Reaching Implications
The dissociation of ionic compounds in water is a fundamental chemical process with wide-ranging implications across numerous disciplines. Understanding the underlying mechanisms, influencing factors, and consequences of this process is crucial for comprehending various natural phenomena and developing technological advancements. From the intricacies of biological systems to the applications in industrial processes, the dissociation of ionic compounds in water remains a cornerstone of chemistry and its applications. Further research into this area continues to unveil new insights and expand its practical relevance.
Latest Posts
Latest Posts
-
How Do You Find The Boiling Point Of A Solution
Apr 02, 2025
-
What Happens To Ionization Energy Down A Group
Apr 02, 2025
-
How Many Grams In A Molecule
Apr 02, 2025
-
Is Cl A Metal Or Nonmetal
Apr 02, 2025
-
Microscopic Anatomy And Organization Of Skeletal Muscle
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Ionic Compounds Dissociate In Water Into . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
