What Type Of Chemical Reaction Is Represented By The Equation
Muz Play
Apr 01, 2025 · 6 min read
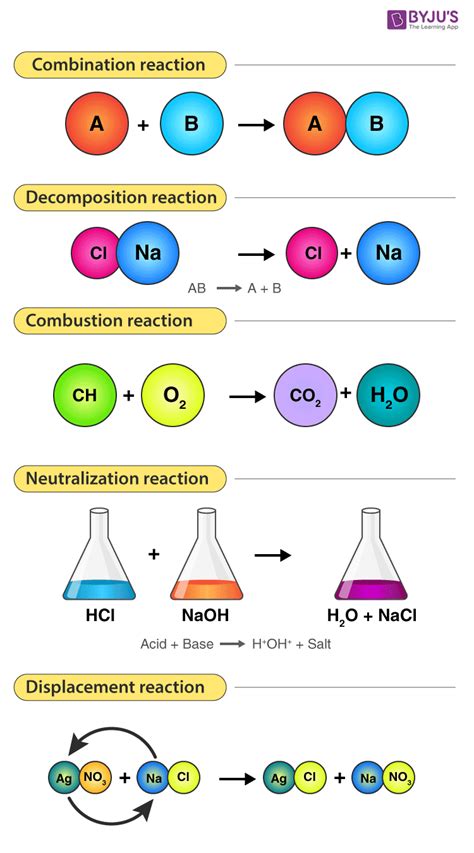
Table of Contents
Deciphering Chemical Equations: A Deep Dive into Reaction Types
Understanding chemical reactions is fundamental to chemistry. A chemical equation, at its core, is a symbolic representation of a chemical reaction, showing the reactants transforming into products. But the type of reaction represented by a specific equation is crucial for predicting outcomes, understanding mechanisms, and applying chemical principles in various fields. This article will explore different types of chemical reactions, providing a detailed analysis of how to classify them based on their equations and the changes they represent. We'll examine various examples, highlighting key characteristics to help you confidently determine the reaction type.
Identifying the Core Reaction Types
Before delving into specifics, let's establish the foundational categories of chemical reactions. These categories are not always mutually exclusive, and some reactions might exhibit characteristics of multiple types. However, understanding these core categories is crucial for analysis.
1. Combination (Synthesis) Reactions:
These reactions involve two or more reactants combining to form a single, more complex product. The general form is:
A + B → AB
Example: The formation of water from hydrogen and oxygen is a classic combination reaction:
2H₂ + O₂ → 2H₂O
Here, hydrogen and oxygen (reactants) combine to form water (product). This is a highly exothermic reaction, releasing significant heat.
Keywords for identification: combine, form, synthesize, single product.
2. Decomposition Reactions:
Decomposition reactions are the opposite of combination reactions. A single reactant breaks down into two or more simpler products. The general form is:
AB → A + B
Example: The decomposition of calcium carbonate upon heating:
CaCO₃ → CaO + CO₂
Calcium carbonate breaks down into calcium oxide and carbon dioxide when heated. This reaction is often endothermic, requiring energy input to proceed.
Keywords for identification: break down, decompose, separate, multiple products from a single reactant.
3. Single Displacement (Substitution) Reactions:
In single displacement reactions, a more reactive element displaces a less reactive element from a compound. The general form is:
A + BC → AC + B
Example: The reaction between zinc and hydrochloric acid:
Zn + 2HCl → ZnCl₂ + H₂
Here, zinc (a more reactive metal) displaces hydrogen from hydrochloric acid, forming zinc chloride and hydrogen gas. The reactivity series of metals is essential for predicting whether a single displacement reaction will occur.
Keywords for identification: displacement, substitution, one element replaces another.
4. Double Displacement (Metathesis) Reactions:
Double displacement reactions involve an exchange of ions between two compounds. The general form is:
AB + CD → AD + CB
Example: The reaction between silver nitrate and sodium chloride:
AgNO₃ + NaCl → AgCl + NaNO₃
Silver nitrate and sodium chloride react to form silver chloride (a precipitate) and sodium nitrate. This reaction is often driven by the formation of a precipitate, a gas, or water.
Keywords for identification: exchange of ions, metathesis, two compounds react to form two new compounds.
5. Combustion Reactions:
Combustion reactions involve the rapid reaction of a substance with oxygen, usually producing heat and light. This is often, but not always, characterized by the presence of oxygen as a reactant. The general form is often, though not always, represented as:
CxHy + O₂ → CO₂ + H₂O (for hydrocarbon combustion)
Example: The combustion of methane:
CH₄ + 2O₂ → CO₂ + 2H₂O
Methane reacts with oxygen to produce carbon dioxide, water, and heat. Complete combustion produces carbon dioxide and water; incomplete combustion may also produce carbon monoxide or soot.
Keywords for identification: burning, combustion, rapid reaction with oxygen, heat and light.
6. Acid-Base Reactions (Neutralization Reactions):
These reactions involve the reaction of an acid and a base to form salt and water. The general form is:
HA + BOH → BA + H₂O
Example: The reaction between hydrochloric acid and sodium hydroxide:
HCl + NaOH → NaCl + H₂O
Hydrochloric acid reacts with sodium hydroxide to form sodium chloride (salt) and water. These reactions are often exothermic.
Keywords for identification: acid, base, neutralization, salt and water formation.
7. Redox Reactions (Oxidation-Reduction Reactions):
Redox reactions involve the transfer of electrons between reactants. One reactant undergoes oxidation (loss of electrons), while another undergoes reduction (gain of electrons). These are often complex and can involve multiple steps.
Example: The reaction between iron and copper(II) sulfate:
Fe + CuSO₄ → FeSO₄ + Cu
Iron loses electrons (oxidation) and copper(II) ions gain electrons (reduction). Identifying redox reactions requires examining oxidation states of elements before and after the reaction.
Keywords for identification: oxidation, reduction, electron transfer, change in oxidation states.
Analyzing a Given Equation to Determine Reaction Type
To determine the type of chemical reaction represented by a specific equation, follow these steps:
-
Identify the reactants and products: Clearly distinguish the substances on the left (reactants) and the right (products) sides of the equation.
-
Examine the number of reactants and products: Are there multiple reactants combining to form a single product (combination), or does a single reactant break down into multiple products (decomposition)?
-
Look for patterns: Does one element replace another (single displacement)? Do ions exchange between two compounds (double displacement)? Is oxygen a reactant, indicating combustion? Is an acid and base reacting (acid-base neutralization)? Are there changes in oxidation states suggesting redox?
-
Consider the overall changes: What are the significant transformations occurring in the reaction? Does the reaction involve the formation of a precipitate, gas, or water? Is there a significant release or absorption of heat?
-
Use keywords: Identifying keywords associated with each reaction type can help in classification.
Example: Let's analyze the equation: 2Na + Cl₂ → 2NaCl
-
Reactants: Sodium (Na) and Chlorine (Cl₂)
-
Products: Sodium chloride (NaCl)
-
Pattern: Two reactants combine to form a single product.
-
Overall change: Sodium and chlorine atoms bond to form an ionic compound.
-
Keywords: Combine, synthesize, form, single product.
Conclusion: This equation represents a combination (synthesis) reaction.
Advanced Considerations and Complex Reactions
Many reactions do not fit neatly into a single category. Some reactions involve multiple reaction steps or exhibit characteristics of several types simultaneously. For instance:
- Multi-step reactions: Some reactions proceed through a series of intermediate steps, each involving a different reaction type. Understanding the mechanism is crucial for accurate classification.
- Concurrent reactions: Several reactions might occur simultaneously in a given system.
- Reactions with multiple products: Some reactions produce multiple products simultaneously, even though the core mechanism belongs to one category.
Advanced techniques, such as analyzing oxidation states and reaction mechanisms, are often necessary to fully classify complex reactions.
Practical Applications and Significance
Understanding chemical reaction types has far-reaching implications across various fields:
- Industrial chemistry: Identifying reaction types is essential for designing efficient and effective industrial processes, optimizing reaction conditions, and predicting yields.
- Environmental chemistry: Classifying reactions helps us understand environmental processes, such as the degradation of pollutants or the formation of smog.
- Biochemistry: Metabolic pathways within living organisms involve numerous reactions, and their classification is critical for understanding cellular processes.
- Analytical chemistry: Knowledge of reaction types allows chemists to devise analytical techniques for identifying and quantifying substances.
By mastering the classification of chemical reactions, you'll significantly enhance your understanding of chemical principles and their diverse applications. Remember that practice is key; the more equations you analyze, the more proficient you'll become in recognizing patterns and identifying reaction types confidently. Always remember to carefully examine the reactants, products, and overall changes to make accurate classifications.
Latest Posts
Latest Posts
-
What Does Fad Stand For In Biology
Apr 02, 2025
-
The Outermost Layer Of The Heart Is Called The
Apr 02, 2025
-
Which Of The Following Are Channels Of Nonverbal Communication
Apr 02, 2025
-
Express The Interval As An Inequality
Apr 02, 2025
-
Toward Or At The Body Surface
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Type Of Chemical Reaction Is Represented By The Equation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
