When Is Work Positive Or Negative Thermodynamics
Muz Play
Apr 06, 2025 · 6 min read
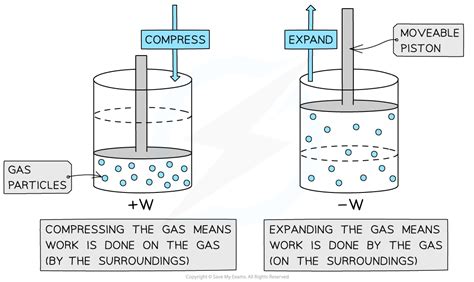
Table of Contents
When is Work Positive or Negative in Thermodynamics?
Thermodynamics, at its core, deals with energy transfer and transformations. A crucial aspect of this is work, defined as energy transferred across a system boundary due to an interaction involving a force and a displacement. However, determining whether work is positive or negative can be tricky, often leading to confusion. This article dives deep into the conventions and applications of positive and negative work in thermodynamics, clarifying the circumstances under which each sign applies. We will explore the impact of system perspectives, different types of work, and the broader implications for thermodynamic processes.
Understanding the Sign Convention: A System's Perspective
The sign convention for work in thermodynamics is inherently tied to the system's perspective. We must always consider whether the work is being done by the system or on the system. This seemingly simple distinction is paramount in correctly assigning the sign.
-
Positive Work (+W): Work is positive when the system does work on its surroundings. In simpler terms, the system is expelling energy in the form of work. Think of a piston expanding and pushing out air – the system (gas in the piston) is performing work on the surroundings (the air outside the piston). Energy is leaving the system.
-
Negative Work (-W): Work is negative when the surroundings do work on the system. The system is receiving energy in the form of work. Imagine compressing a spring – the surroundings (your hand) are doing work on the system (the spring). Energy is entering the system.
This system-centric approach is vital because the same physical process can yield opposite signs depending on which component you define as the system. For example, consider a weight being lifted. If the weight is the system, the work is negative (work is being done on the weight). Conversely, if the lifting mechanism is the system, the work is positive (the mechanism is doing work on the weight).
Different Types of Work and their Sign Conventions
While the fundamental principle remains the same (work done by the system is positive, work done on the system is negative), various types of work present unique scenarios:
1. Pressure-Volume Work (Expansion/Compression):
This is the most common type of work encountered in thermodynamics, usually associated with gases. It's calculated as:
W = -∫PdV
where:
- W = work done
- P = pressure
- V = volume
The negative sign stems from the convention established: when the gas expands (dV is positive), the work done by the system is positive according to the equation. Conversely, when the gas is compressed (dV is negative), work is done on the system and work becomes positive. This equation ensures the alignment with our fundamental system-centric convention.
Positive Pressure-Volume Work: Occurs during expansion of a gas against an external pressure. The system loses energy.
Negative Pressure-Volume Work: Occurs during the compression of a gas. The system gains energy.
2. Electrical Work:
Electrical work involves the movement of charge under the influence of a potential difference.
Positive Electrical Work: The system performs electrical work on the surroundings, such as in a battery discharging. The system loses energy.
Negative Electrical Work: The surroundings perform electrical work on the system, for instance, when charging a capacitor. The system gains energy.
3. Shaft Work:
This type of work involves rotational motion, often encountered in engines and turbines.
Positive Shaft Work: The system is doing work, like a rotating turbine driving a generator. Energy leaves the system.
Negative Shaft Work: Work is done on the system, for example, a motor driving a shaft. Energy enters the system.
4. Surface Work:
Surface work concerns the changes in surface area.
Positive Surface Work: The system increases its surface area (like a liquid spreading), thus doing work on the surroundings. Energy leaves the system.
Negative Surface Work: The system decreases its surface area, and the surroundings do work on the system. Energy enters the system.
Beyond the Sign: Implications for Thermodynamic Processes
The sign of work is not just a matter of convention; it's profoundly linked to the overall energy balance of a thermodynamic system. Positive work indicates that the system is losing internal energy, while negative work implies a gain in internal energy. This connection is crucial when applying the First Law of Thermodynamics:
ΔU = Q + W
where:
- ΔU = change in internal energy of the system
- Q = heat transferred to the system
- W = work done on or by the system
This equation underscores the interconnectedness of heat, work, and internal energy. The sign of W directly influences the change in internal energy (ΔU). A positive W (work done by the system) leads to a decrease in internal energy, assuming Q is constant or less. A negative W (work done on the system) increases the internal energy.
Analyzing thermodynamic processes involves determining not only the magnitude of work but also its sign. This determines if a process is spontaneous or requires external input, and whether the system is gaining or losing energy.
Examples Illustrating Positive and Negative Work:
Let's consider a few practical examples to solidify our understanding:
Example 1: Isothermal Expansion of an Ideal Gas
An ideal gas expands isothermally against a constant external pressure. The gas is the system. Since the gas expands, it is doing work on the surroundings. This means W is positive.
Example 2: Adiabatic Compression of a Gas
A gas is compressed adiabatically (no heat transfer). The gas is the system. Because the surroundings are doing work on the gas, W is negative.
Example 3: A Battery Powering a Motor
A battery is connected to a motor, causing the motor to rotate. The battery is the system. The battery is performing work on the motor, causing a loss of chemical energy. W is positive.
Practical Applications and Real-World Scenarios
The concepts of positive and negative work are not merely theoretical. They hold immense practical importance across various fields:
-
Engineering: Designing efficient engines, turbines, and other machines requires careful consideration of work done by and on the system. Optimizing these processes requires a clear understanding of work's sign to maximize energy output.
-
Chemistry: Understanding work done during chemical reactions, such as in electrochemical cells, helps predict spontaneity and efficiency.
-
Environmental Science: Studying work done in natural processes, like atmospheric circulation and geological formations, is fundamental to understanding the energy balance in the environment.
-
Material Science: Analyzing the mechanical work involved in material deformation or processing is key to developing and manipulating new materials with specific properties.
Conclusion: Mastering the Nuances of Work in Thermodynamics
The determination of whether work is positive or negative in thermodynamics is not a trivial aspect but a crucial element for accurately analyzing and interpreting energy transfer. By firmly grasping the system perspective and consistently applying the sign convention, we can gain a deeper understanding of energy transformations in various thermodynamic processes. This comprehensive understanding extends far beyond theoretical exercises and finds direct applications in a wide range of scientific and engineering disciplines. Remember, always focus on who is doing the work – the system or its surroundings – to correctly assign the sign of W. A proper understanding of this convention is essential for accurate thermodynamic calculations and a thorough understanding of energy transfer and transformations.
Latest Posts
Latest Posts
-
What Is The Special Property Of Water
Apr 09, 2025
-
What Is A Titrant In Chemistry
Apr 09, 2025
-
What Does A High Shannon Diversity Index Mean
Apr 09, 2025
-
What Chemical Binds Free Hydrogen Ions In Solution
Apr 09, 2025
-
Biologists Use The Fluid Mosaic Model To Describe Membrane Structure
Apr 09, 2025
Related Post
Thank you for visiting our website which covers about When Is Work Positive Or Negative Thermodynamics . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.