When Two Amino Acids Are Joined Together
Muz Play
Mar 18, 2025 · 6 min read
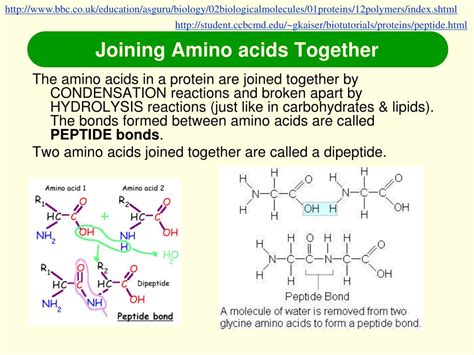
Table of Contents
When Two Amino Acids Are Joined Together: Peptide Bond Formation and Its Significance
The fundamental building blocks of proteins are amino acids. These aren't simply strung together haphazardly; their connection is a precisely orchestrated chemical reaction resulting in a strong, stable bond crucial for protein structure and function. Understanding how two amino acids join together – a process involving peptide bond formation – is key to grasping the complexities of biochemistry and the life sciences. This article will delve deep into the mechanism, implications, and significance of this vital process.
The Chemistry of Peptide Bond Formation
Amino acids possess a characteristic structure featuring an amino group (-NH₂), a carboxyl group (-COOH), a hydrogen atom (-H), and a unique side chain (R-group) all bonded to a central carbon atom (the α-carbon). The diversity of the R-group is what defines the 20 standard amino acids. It's the carboxyl group of one amino acid and the amino group of another that participate in the creation of a peptide bond.
The Condensation Reaction
Peptide bond formation is a condensation reaction, also known as a dehydration reaction. This means that a molecule of water is released during the bond's creation. Specifically:
- The hydroxyl group (-OH) from the carboxyl group of one amino acid and a hydrogen atom (-H) from the amino group of the other amino acid combine to form water (H₂O).
- The remaining carbon atom (C) of the carboxyl group and the nitrogen atom (N) of the amino group form a covalent bond – the peptide bond (also called an amide bond).
This process requires energy, typically provided by ATP (adenosine triphosphate) within the cellular environment. The reaction is catalyzed by ribosomes in the process of protein synthesis, and by specific enzymes in other contexts.
The Peptide Bond: A Unique Covalent Link
The peptide bond itself is a unique type of covalent bond with several key characteristics:
- Planar Geometry: The six atoms participating in the peptide bond (C=O, N-H, and the α-carbons of the two amino acids) lie in a single plane. This planarity is due to resonance stabilization involving the carbonyl group.
- Partial Double Bond Character: The resonance between the carbonyl oxygen and the amide nitrogen gives the peptide bond a partial double bond character. This restricts rotation around the peptide bond, influencing protein conformation.
- Polarity: The peptide bond is polar due to the electronegativity difference between the oxygen and nitrogen atoms. This polarity influences protein folding and interactions with water molecules.
- Trans Configuration: In most naturally occurring peptides, the amino acid side chains are positioned on opposite sides of the peptide bond (trans configuration). This is the more energetically favorable configuration.
The Process of Peptide Bond Formation: A Step-by-Step Look
Let's visualize the process using two simple amino acids, glycine (Gly) and alanine (Ala):
-
Approach: The carboxyl group of glycine approaches the amino group of alanine. This proximity is facilitated by the cellular environment and enzymatic activity.
-
Activation: The carboxyl group of glycine is activated, often through the attachment of a high-energy molecule like ATP. This increases its reactivity.
-
Nucleophilic Attack: The nitrogen atom of alanine's amino group acts as a nucleophile, attacking the electrophilic carbon atom of glycine's carboxyl group.
-
Tetrahedral Intermediate: A transient tetrahedral intermediate is formed. This intermediate is unstable.
-
Water Removal: A proton (H⁺) is transferred, and a water molecule is eliminated.
-
Peptide Bond Formation: The carbon atom of glycine's carboxyl group and the nitrogen atom of alanine's amino group form a covalent bond—the peptide bond. A dipeptide (Gly-Ala) is now formed.
-
Product Release: The newly formed dipeptide is released from the enzyme or ribosome.
Extending the Chain: Polypeptides and Proteins
The process isn't limited to two amino acids. The carboxyl group of the newly formed dipeptide can react with the amino group of another amino acid, extending the chain to form a tripeptide, then a tetrapeptide, and so on. This continues until a long chain of amino acids is produced, called a polypeptide. When a polypeptide chain folds into a specific three-dimensional structure, it becomes a protein. The sequence of amino acids, their specific R-groups, and the subsequent folding determine the protein's unique function.
The Role of Enzymes and Ribosomes
The formation of peptide bonds in biological systems is highly efficient and regulated. This is primarily due to the involvement of specialized enzymes and cellular machinery:
-
Ribosomes: These are complex molecular machines within cells that are responsible for protein synthesis. They catalyze peptide bond formation during translation, the process of converting the genetic code into a polypeptide chain. Ribosomal RNA (rRNA) plays a crucial catalytic role in this process.
-
Peptidyl Transferase: This enzyme, part of the ribosome, specifically catalyzes peptide bond formation between amino acids linked to transfer RNA (tRNA) molecules.
-
Other Enzymes: In certain processes outside protein synthesis, specific enzymes can catalyze peptide bond formation. These often play a role in modifying existing proteins or creating small peptides with specific functions.
Significance of Peptide Bond Formation
The ability of amino acids to join together to form peptide bonds is paramount to life as we know it. The implications are vast and extend to numerous aspects of biology:
-
Protein Synthesis: Peptide bond formation is the cornerstone of protein synthesis, the fundamental process through which cells build proteins needed for a myriad of functions.
-
Protein Structure and Function: The sequence of amino acids linked by peptide bonds dictates the primary structure of a protein. This, in turn, influences higher-order structures (secondary, tertiary, and quaternary) which determine protein function.
-
Enzyme Activity: Many enzymes are proteins, and their catalytic activities rely on their precise three-dimensional structures formed through peptide bond linkages.
-
Hormone Production: Many hormones are peptide or protein hormones, synthesized through the linking of amino acids.
-
Immune System: Antibodies, crucial components of the immune system, are proteins with specific structures shaped by peptide bonds.
-
Cellular Signaling: Peptide hormones and other signaling molecules use peptide bonds to form their functional structures.
-
Structural Proteins: Structural proteins like collagen and keratin rely heavily on peptide bonds for their strength and stability.
Understanding Peptide Bond Hydrolysis
The reverse of peptide bond formation is peptide bond hydrolysis. This is the breaking of a peptide bond through the addition of a water molecule. This process is essential for protein degradation, allowing cells to recycle amino acids and regulate protein levels. Hydrolysis can occur spontaneously but is significantly accelerated by enzymes called proteases or peptidases.
Conclusion: A Foundation of Life
The formation of a peptide bond, a seemingly simple chemical reaction, is a remarkably significant process. Its precision and efficiency are essential for life itself. The robust yet versatile nature of the peptide bond allows for the creation of a vast array of proteins with diverse structures and functions, making it a fundamental concept in biochemistry and molecular biology. Further research into the mechanisms and regulation of peptide bond formation continues to unveil new insights into biological processes and potential therapeutic targets. This understanding is crucial for advancements in various fields, from developing new drugs to engineering novel protein-based materials.
Latest Posts
Latest Posts
-
Converting Double Integrals To Polar Coordinates
Mar 19, 2025
-
How Do You Convert Moles To Volume
Mar 19, 2025
-
The Horizontal Columns On The Periodic Table Are Called
Mar 19, 2025
-
How To Find Derivative Of Limit
Mar 19, 2025
-
Proof Of The Parallel Axis Theorem
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about When Two Amino Acids Are Joined Together . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
