Where Are Prolines Found On An Alpha Helix
Muz Play
Mar 21, 2025 · 6 min read
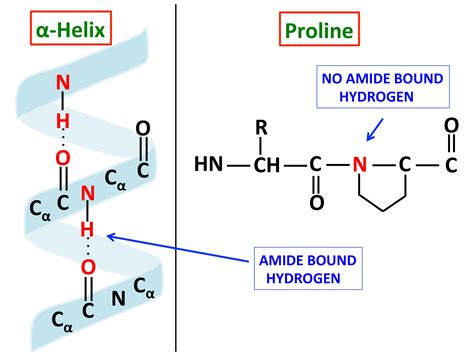
Table of Contents
Where are Prolines Found on an Alpha Helix? The Role of Proline in Protein Structure
Proline, often considered the "helix breaker," plays a unique and complex role in protein structure. While it disrupts the typical α-helix structure, its presence isn't entirely disruptive; instead, it introduces specific conformational changes and influences the overall protein folding. Understanding where proline residues are found on an α-helix, and the implications of their placement, is crucial for comprehending protein function and stability. This article delves into the intricacies of proline's interaction with α-helices, exploring its impact on helix stability, its location preferences, and the consequences for protein structure and function.
Understanding the Alpha Helix Structure
Before examining proline's influence, let's briefly review the α-helix structure. The α-helix is a common secondary structure element in proteins, characterized by a right-handed coiled conformation. This structure is stabilized by hydrogen bonds between the carbonyl oxygen of one amino acid residue and the amide hydrogen of the residue four positions down the sequence (n and n+4). This specific hydrogen bonding pattern gives rise to the characteristic helical structure with approximately 3.6 amino acid residues per turn.
The α-carbon atoms of the amino acids lie along the axis of the helix, while the side chains extend outward. The peptide backbone is tightly wound, creating a relatively rigid rod-like structure. The stability of the α-helix is influenced by several factors, including the amino acid sequence, the solvent environment, and the presence of specific amino acid residues such as proline.
Proline: The Helix Breaker and Its Unique Properties
Proline is an imino acid, unlike other amino acids which are α-amino acids. This structural difference stems from proline's cyclic structure, where the side chain is bonded to both the α-carbon and the nitrogen atom, forming a rigid five-membered ring. This cyclic structure restricts the conformational flexibility of proline, significantly influencing its ability to participate in α-helix formation.
The Impact on Hydrogen Bonding: The rigid nature of proline's ring prevents the formation of the typical hydrogen bond between its own amide nitrogen and the carbonyl group of the residue four positions down the sequence. This absence of a crucial hydrogen bond disrupts the regular hydrogen bonding pattern essential for α-helix stability. As a result, proline is often found at the ends of α-helices or occasionally within the helix, creating a kink or bend.
Steric Hindrance: Proline's bulky side chain also introduces steric hindrance, further impeding the formation of a stable α-helix. The side chain's close proximity to the main chain restricts the rotational freedom of the peptide backbone, leading to deviations from the ideal α-helix geometry.
Where Proline is Typically Found in α-Helices
While often referred to as a "helix breaker," proline isn't always disruptive. Its position within a protein significantly affects its impact on the α-helix. Here's a breakdown of common locations:
1. At the N-Terminus of an α-Helix:
Proline is frequently found at the N-terminus of α-helices. In this position, it lacks the ability to form the crucial hydrogen bond with the residue four positions down the sequence, but it does not significantly affect the stability of the rest of the helix. The absence of this initial hydrogen bond may be less detrimental than in the middle of the helix.
2. At the C-Terminus of an α-Helix:
Similarly, proline can be located at the C-terminus. Here, the lack of a hydrogen bond involving proline’s amide nitrogen doesn't destabilize the preceding part of the helix significantly. The C-terminus is inherently less constrained, so the disruptive effect of proline is minimized.
3. Within the α-Helix:
While less common, proline can occasionally be found within an α-helix. In such instances, it often introduces a kink or bend in the helix, resulting in a localized disruption of the helical structure. The degree of disruption depends on factors like the surrounding amino acids and the protein's overall conformation. This kink can be functionally important, especially in situations where the protein needs to undergo conformational changes or interact with other molecules.
4. Influence of neighboring residues:
The effect of a proline residue on helix stability isn't solely dependent on its position; the surrounding amino acid sequence also plays a critical role. For example, the presence of certain amino acids near proline can compensate for its helix-breaking tendencies. Conversely, certain residues can enhance proline's disruptive effect.
The Functional Significance of Proline's Placement
The strategic placement of proline isn't random. Its presence in specific locations often plays crucial roles in protein function and stability.
1. Introducing Turns and Kinks:
Proline's ability to disrupt the α-helix structure enables the formation of turns and kinks necessary for proteins to adopt their complex three-dimensional shapes. These turns and kinks are critical for protein folding and function.
2. Mediating Protein-Protein Interactions:
The kink or bend introduced by proline can create a surface conducive to protein-protein interactions. This altered surface can facilitate binding to other proteins or molecules, which is vital for various cellular processes.
3. Stabilizing Specific Conformations:
In some cases, proline contributes to the stabilization of specific protein conformations, even within α-helices. Although it disrupts typical hydrogen bonding patterns, the inherent rigidity of its structure can sometimes contribute to the overall stability of the protein.
4. Influence on Membrane Proteins:
Proline's presence is particularly notable in membrane proteins. Its rigid structure and ability to introduce bends are often crucial for anchoring the protein within the lipid bilayer and maintaining its structural integrity in this hydrophobic environment. The specific placement of proline can influence how the protein interacts with the membrane.
Predicting Proline's Location: Computational Tools and Methods
Predicting the location and influence of proline in proteins is a significant area of bioinformatics research. Several computational tools and methods are employed to analyze protein sequences and predict the secondary structure, including the presence and location of α-helices and the likely disruption caused by proline residues. These tools often utilize algorithms that consider factors like amino acid sequence, hydrogen bonding potential, and steric interactions.
While these tools provide valuable insights, it's crucial to remember that they are predictive and not definitive. Experimental techniques like X-ray crystallography and NMR spectroscopy remain essential for confirming the precise three-dimensional structure and identifying the exact role of proline residues.
Conclusion: Proline's Versatile Role in Protein Structure
Proline, far from being simply a "helix breaker," plays a multifaceted and crucial role in shaping the structure and function of proteins. Its location within α-helices is not random but strategically placed to influence protein folding, stability, and interaction with other molecules. While it disrupts the typical α-helix structure through its unique cyclic nature and its inability to participate in typical hydrogen bonding, its presence is often necessary for protein function. Understanding where proline is found and how it influences the protein's conformation remains an active area of research with implications for numerous fields, including drug design, protein engineering, and the understanding of disease mechanisms. Further research into the intricate interplay between proline and α-helices will undoubtedly continue to deepen our understanding of protein structure and function.
Latest Posts
Latest Posts
-
Standard Error For Difference In Means
Mar 22, 2025
-
Definition Of Reference Group In Sociology
Mar 22, 2025
-
The Study Of The Geographical Distribution Of Organisms Is Called
Mar 22, 2025
-
Lewis Dot Structures For Polyatomic Ions
Mar 22, 2025
-
Is Tap Water A Homogeneous Mixture
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about Where Are Prolines Found On An Alpha Helix . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
