Where Are The Nonmetals Located On The Periodic Table
Muz Play
Mar 23, 2025 · 5 min read
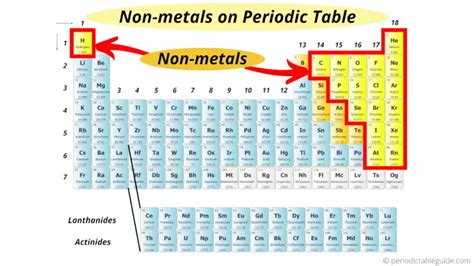
Table of Contents
Where Are the Nonmetals Located on the Periodic Table? A Comprehensive Guide
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. Understanding the placement of elements allows us to predict their behavior and reactivity. This article delves into the location of nonmetals on the periodic table, exploring their properties, characteristics, and the trends that govern their placement. We will also explore some exceptions and interesting nuances within this category of elements.
Defining Nonmetals: Properties and Characteristics
Before we pinpoint their location, let's clarify what constitutes a nonmetal. Nonmetals are elements that generally lack metallic properties. This means they typically exhibit the following:
-
Poor conductors of heat and electricity: Unlike metals, nonmetals are generally poor conductors, meaning they don't readily allow heat or electricity to pass through them. This is due to their electronic structure and the way electrons are bound within their atoms.
-
Brittle and non-ductile: Most nonmetals are brittle, meaning they shatter easily when subjected to stress. They are not easily drawn into wires (ductile) or hammered into sheets (malleable) like metals.
-
Low melting and boiling points: Compared to metals, nonmetals tend to have significantly lower melting and boiling points. This is a reflection of the weaker interatomic forces holding their atoms together.
-
Gain electrons easily: Nonmetals readily gain electrons to achieve a stable electron configuration, often forming negative ions (anions). This tendency drives their chemical reactivity.
-
Variety of physical states: Nonmetals exist in various physical states at room temperature. For example, oxygen and nitrogen are gases, bromine is a liquid, and iodine is a solid. This diversity contrasts with the mostly solid nature of metals.
The Geographic Location of Nonmetals on the Periodic Table
Nonmetals are located primarily in the upper right-hand corner of the periodic table. They are separated from the metals by a diagonal line running from boron (B) to astatine (At). Elements bordering this line, known as metalloids or semimetals, exhibit properties intermediate between metals and nonmetals.
This seemingly simple statement belies the rich diversity within the nonmetal group. Let's break down the specific locations more precisely:
Group 17 (Halogens)
The halogens (fluorine, chlorine, bromine, iodine, and astatine) occupy Group 17 of the periodic table. These highly reactive elements are known for their ability to readily gain one electron to form a stable negative ion with a -1 charge. Their reactivity decreases as we move down the group. Fluorine, the most electronegative element, is exceptionally reactive.
Group 16 (Chalcogens)
Group 16, also known as the chalcogens (oxygen, sulfur, selenium, tellurium, and polonium), sits to the left of the halogens. These elements typically gain two electrons to achieve a stable electron configuration. Oxygen is crucial for respiration, while sulfur plays a vital role in various biological processes.
Group 15 (Pnictogens)
The pnictogens (nitrogen, phosphorus, arsenic, antimony, and bismuth) reside in Group 15. Their reactivity varies, with nitrogen being relatively unreactive under normal conditions, while phosphorus exists in multiple allotropes with different reactivities.
Group 14 (Tetrels)
Carbon, silicon, germanium, tin, and lead make up Group 14. While carbon is undeniably a nonmetal, the metallic character increases as we move down the group. Tin and lead exhibit significant metallic properties. Carbon's unique ability to form long chains and complex structures is fundamental to organic chemistry and the existence of life as we know it.
Group 13 (Icosagens)
Boron, the only nonmetal in Group 13, is a unique element with properties that bridge the gap between metals and nonmetals. It’s often considered a metalloid due to its semi-conducting properties.
Hydrogen (A Unique Case)
Hydrogen, located in Group 1, presents a special case. While it possesses some properties of nonmetals, its placement is often debated. Its single electron and ability to readily form both positive and negative ions make it unique and somewhat distinct from other nonmetals.
Trends in Nonmetal Properties Across the Periodic Table
Understanding the trends in nonmetal properties as we move across and down the periodic table provides further insight into their location and behavior:
Across a Period (Left to Right):
-
Electronegativity increases: As we move across a period from left to right, the electronegativity of nonmetals increases. This means their ability to attract electrons in a chemical bond increases.
-
Ionization energy increases: The energy required to remove an electron from a nonmetal atom also increases as we move across a period.
-
Atomic radius decreases: The size of the atoms generally decreases as we go from left to right across a period.
Down a Group (Top to Bottom):
-
Electronegativity decreases: As we move down a group, the electronegativity of nonmetals generally decreases.
-
Ionization energy decreases: The energy required to remove an electron decreases as we move down a group.
-
Atomic radius increases: The size of the atoms increases as we move down a group.
Exceptions and Nuances
The periodic table is a model, and like all models, it has its limitations. Some elements might show properties that deviate from the general trends, leading to some ambiguity in classification. For instance, some elements near the metalloid line might display properties of both metals and nonmetals depending on the conditions.
The Importance of Understanding Nonmetal Location
Knowing where nonmetals are located on the periodic table is crucial for several reasons:
-
Predicting chemical behavior: The location of an element provides clues about its reactivity, bonding patterns, and the types of compounds it will form.
-
Understanding chemical reactions: Knowing the properties of nonmetals helps in predicting the outcome of chemical reactions involving these elements.
-
Developing new materials: The unique properties of nonmetals make them essential in the design and development of new materials with specific applications. For example, the semiconducting properties of some nonmetals are crucial in electronics.
Conclusion: A Systematic Approach to Understanding Nonmetals
The location of nonmetals on the periodic table is not arbitrary; it reflects fundamental trends in their atomic structure and properties. By understanding these trends and the exceptions to the rules, we can better appreciate the diverse roles nonmetals play in our world. From the air we breathe (oxygen and nitrogen) to the materials we use every day (carbon in plastics and silicon in computer chips), nonmetals are integral to our lives and understanding their position on the periodic table is key to unlocking their potential. Further exploration of individual nonmetals and their unique characteristics will only deepen our appreciation of their importance in chemistry and beyond. Remember, the periodic table is a powerful tool; learning to navigate it effectively unlocks a wealth of chemical knowledge.
Latest Posts
Latest Posts
-
What The Purpose Of Narrative Writing
Mar 25, 2025
-
What Is Ph At The Equivalence Point
Mar 25, 2025
-
How Do The Nucleus And Ribosomes Work Together
Mar 25, 2025
-
A Measure Of The Amount Of Matter
Mar 25, 2025
-
Area Of A Surface Of Revolution Formula
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Where Are The Nonmetals Located On The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
