Where Are The Reactants Located In A Chemical Equation
Muz Play
Mar 19, 2025 · 5 min read
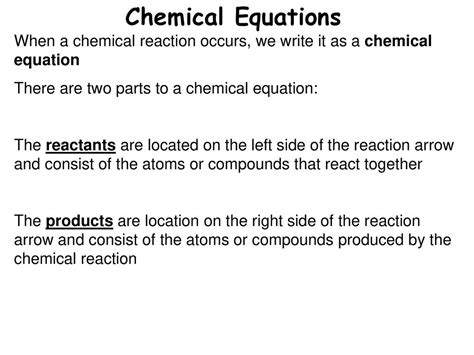
Table of Contents
Where Are the Reactants Located in a Chemical Equation? A Comprehensive Guide
Understanding the location of reactants in a chemical equation is fundamental to grasping the basics of chemistry. This seemingly simple question opens the door to a deeper understanding of chemical reactions, stoichiometry, and the language of chemistry itself. This comprehensive guide will explore the placement of reactants within chemical equations, delve into the underlying principles, and provide practical examples to solidify your comprehension.
The Anatomy of a Chemical Equation: Identifying Reactants and Products
Before pinpointing the location of reactants, let's establish what a chemical equation represents. A chemical equation is a concise way to describe a chemical reaction, using chemical formulas and symbols to represent the substances involved. It follows a specific format:
Reactants → Products
This simple arrow signifies the transformation of reactants into products. The reactants are the starting materials, the substances that undergo a chemical change. The products are the resulting substances formed after the reaction.
Reactants: The Starting Point of Chemical Transformations
The reactants are always found on the left-hand side of the chemical equation. They are listed with their respective chemical formulas, separated by plus signs (+) indicating their involvement in the reaction. The number of molecules or moles of each reactant is represented by coefficients placed before the chemical formula. These coefficients are crucial for balancing the equation, ensuring the law of conservation of mass is upheld.
Example: The combustion of methane (CH₄) with oxygen (O₂) to produce carbon dioxide (CO₂) and water (H₂O) is represented as:
CH₄ + 2O₂ → CO₂ + 2H₂O
In this equation, CH₄ and 2O₂ are the reactants, located on the left side of the arrow. The '2' before O₂ indicates two molecules of oxygen gas are required for the reaction to proceed completely.
The Importance of Reactant Placement: Understanding the Reaction Process
The consistent placement of reactants on the left side of the equation is not arbitrary; it's a standardized convention that provides clarity and facilitates understanding of the chemical process. This convention allows chemists worldwide to communicate effectively and unambiguously about chemical reactions.
Visualizing the Reaction:
Imagine the reaction as a transformation process. The reactants, positioned on the left, are like the ingredients in a recipe. They are the initial components that enter the chemical "oven" (the reaction conditions). Through the reaction process, they undergo a change in chemical composition and properties, transforming into the products on the right.
Predicting Reaction Outcomes:
Knowing the location of reactants enables us to predict potential reaction outcomes. By analyzing the reactants and their properties, chemists can use their understanding of chemical principles to foresee the type of products that might form and the reaction's overall direction.
Balancing Equations and Stoichiometry:
The placement of reactants is fundamental to balancing chemical equations, a critical aspect of stoichiometry. Balancing involves adjusting the coefficients in front of the reactants and products to ensure an equal number of atoms of each element on both sides of the equation. This ensures that mass is conserved during the chemical reaction, a cornerstone of chemical principles.
Beyond the Basics: Complex Reaction Scenarios
While the basic format outlined above holds true for most reactions, some scenarios require a nuanced understanding.
Reversible Reactions:
Reversible reactions are characterized by an equilibrium between reactants and products. The reaction proceeds in both directions, with reactants forming products and vice versa. These are often represented with a double arrow (⇌). Reactants are still located on the left side, but their conversion to products is not unidirectional.
Example: The reaction between nitrogen and hydrogen to form ammonia:
N₂ + 3H₂ ⇌ 2NH₃
Here, N₂ and 3H₂ are the reactants on the left. However, the double arrow signifies that ammonia can also decompose back into nitrogen and hydrogen.
Multi-step Reactions:
Some chemical reactions involve multiple steps. These multi-step reactions can be broken down into a series of simpler reactions, each with its own set of reactants and products. The overall reaction incorporates all the steps. Reactants in the overall reaction are those initially involved in the first step. Intermediate products formed in intermediate steps might serve as reactants in subsequent steps.
Reaction Mechanisms:
A reaction mechanism describes the precise sequence of elementary steps that constitute a complex reaction. While the overall reaction shows reactants and products, the mechanism details the individual steps, showing intermediate species and transition states that may not be explicitly included in the overall balanced equation. The reactants of each elementary step are still identifiable.
Practical Applications and Real-World Examples
Understanding reactant placement has crucial applications in various fields:
- Industrial Chemistry: Determining the optimal ratio of reactants is essential for maximizing product yield and efficiency in industrial chemical processes.
- Environmental Science: Analyzing pollutants and their reactions in the environment involves identifying reactants to predict the potential environmental impact and devise remediation strategies.
- Medicine: Understanding chemical reactions in biological systems, such as drug metabolism, requires knowledge of the reactants involved in various biochemical pathways.
Let's explore a few real-world examples:
1. Photosynthesis: The reactants for photosynthesis – carbon dioxide (CO₂) and water (H₂O) – are on the left of the equation:
6CO₂ + 6H₂O + Light Energy → C₆H₁₂O₆ + 6O₂
2. Rusting of Iron: The reactants involved in the rusting of iron are iron (Fe) and oxygen (O₂):
4Fe + 3O₂ + 6H₂O → 4Fe(OH)₃
3. Neutralization Reactions: Acid-base neutralization reactions involve a reaction between an acid (e.g., HCl) and a base (e.g., NaOH):
HCl + NaOH → NaCl + H₂O
Conclusion: Mastering the Language of Chemistry
The simple convention of placing reactants on the left-hand side of a chemical equation is a crucial element in chemical notation. This consistent placement makes it possible to quickly identify starting materials in any chemical reaction, regardless of its complexity. By grasping this fundamental concept, you lay the foundation for understanding chemical processes, predicting reaction outcomes, and ultimately, mastering the language of chemistry. This knowledge is essential for anyone studying chemistry, from beginners to advanced researchers, paving the way for deeper explorations into the fascinating world of chemical reactions and their applications. Remember to practice balancing equations and working through various examples to solidify your understanding. The consistent practice will allow you to quickly identify reactants and products in any given chemical reaction.
Latest Posts
Latest Posts
-
Label The Veins Of The Lower Limb
Mar 19, 2025
-
How To Find Change In Enthalpy From Q
Mar 19, 2025
-
Large Size Crystals Are Know As Phaneritic Are Called
Mar 19, 2025
-
How To Identify Weak And Strong Acids And Bases
Mar 19, 2025
-
Do Protons Have About The Same Mass As Neutrons
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Where Are The Reactants Located In A Chemical Equation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
