Which Change Of Phase Is Exothermic
Muz Play
Mar 20, 2025 · 6 min read
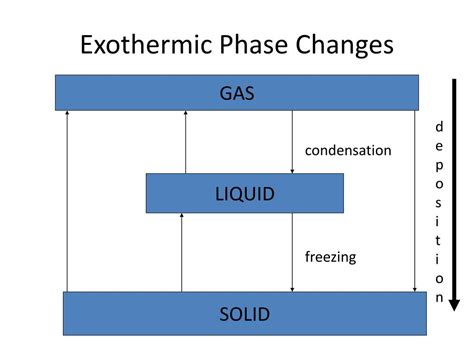
Table of Contents
Which Change of Phase is Exothermic? Understanding Phase Transitions and Heat Flow
Phase transitions, the processes by which matter changes from one state to another (like solid to liquid, liquid to gas, etc.), are fundamental concepts in chemistry and physics. Understanding whether a phase change is exothermic (releases heat) or endothermic (absorbs heat) is crucial for many applications, from industrial processes to weather forecasting. This article will delve into the specifics of exothermic phase transitions, exploring the underlying principles and providing examples to solidify your understanding.
Understanding Exothermic Processes
Before diving into specific phase changes, let's establish a clear understanding of what constitutes an exothermic process. Exothermic reactions and processes are characterized by the release of heat to the surroundings. This means the system (the substance undergoing the phase change) loses energy, and the surroundings gain energy, resulting in an increase in the temperature of the environment. The opposite, an endothermic process, involves the absorption of heat from the surroundings.
The energy change during a phase transition is directly related to the intermolecular forces between the particles (atoms, molecules, or ions) of the substance. These forces hold the particles together, and the strength of these forces dictates the amount of energy required to overcome them and change the phase.
Exothermic Phase Transitions: A Closer Look
While many associate phase changes with endothermic processes (like melting ice), several phase transitions are exothermic. The key is to focus on the direction of the phase change. Exothermic phase transitions always involve a decrease in the energy of the system. This typically means moving from a higher-energy state to a lower-energy state. The most common exothermic phase changes are:
1. Condensation: Gas to Liquid
Condensation is perhaps the most readily apparent example of an exothermic phase change. When a gas transitions into a liquid state, the particles lose kinetic energy, and the intermolecular forces between them become stronger. This energy is released as heat into the surroundings.
Mechanism: In a gas, particles are far apart and move rapidly with high kinetic energy. As the gas cools, the particles slow down, and the attractive intermolecular forces begin to dominate. These forces pull the particles closer together, forming a liquid. The energy lost during this process is released as heat.
Examples:
- Dew formation: Water vapor in the air cools down overnight, condensing into liquid water on surfaces.
- Fog formation: Water vapor condenses into tiny liquid water droplets suspended in the air.
- Rain formation: Water vapor in clouds condenses into larger droplets, eventually falling as rain.
- Steam condensing on a cold surface: When steam comes into contact with a cooler surface, it loses energy and condenses into liquid water, releasing heat to the surface.
2. Deposition: Gas to Solid
Deposition is a less common but equally important exothermic phase transition. In this process, a gas directly transitions into a solid state without passing through the liquid phase. This occurs when the gas is cooled significantly, causing the particles to lose kinetic energy and directly form a solid structure.
Mechanism: Similar to condensation, deposition involves a decrease in kinetic energy and an increase in the strength of intermolecular forces. The energy released during this transition is released to the surroundings.
Examples:
- Frost formation: Water vapor in the air directly deposits as ice crystals on cold surfaces.
- Snow formation: Water vapor in clouds can directly deposit as snow crystals under specific atmospheric conditions.
- Formation of ice crystals in freezers: Water vapor in a freezer can directly deposit as ice crystals on frozen food.
3. Freezing: Liquid to Solid
Freezing is another familiar example of an exothermic phase change. As a liquid cools, the kinetic energy of its particles decreases. The attractive intermolecular forces become stronger, causing the particles to arrange themselves in a more ordered, solid structure. This ordering process releases energy as heat.
Mechanism: In the liquid state, particles have more freedom of movement than in the solid state. As the liquid cools, this freedom decreases, and the particles settle into fixed positions within a crystal lattice. The energy released during this ordering process is heat.
Examples:
- Water freezing into ice: A classic example, as liquid water releases heat as it freezes into ice.
- Molten metal solidifying: When molten metals cool, they release heat as they solidify into their solid crystalline forms.
- Candle wax solidifying: As liquid candle wax cools, it solidifies, releasing heat into the surrounding environment.
Factors Affecting Exothermic Phase Transitions
Several factors influence the rate and extent of exothermic phase transitions:
-
Temperature: Lower temperatures generally favor exothermic phase transitions. The lower the temperature, the less kinetic energy the particles possess, making it easier for intermolecular forces to dominate and facilitate the phase change.
-
Pressure: Pressure can also influence exothermic phase transitions, particularly condensation and deposition. Higher pressures generally favor the condensed phases (liquid and solid) as they compress the particles closer together, increasing the strength of intermolecular forces.
-
Impurities: The presence of impurities in a substance can affect the freezing point and the rate of freezing, influencing the exothermic heat released during the transition.
Applications of Exothermic Phase Transitions
Exothermic phase transitions play crucial roles in various applications:
-
Refrigeration and air conditioning: These systems rely on the exothermic phase transitions of refrigerants to cool the environment. The refrigerant absorbs heat when it evaporates (endothermic) and releases heat when it condenses (exothermic).
-
Industrial processes: Many industrial processes involve controlled phase transitions, such as the solidification of metals or the condensation of vapors, with exothermic heat release needing to be managed.
-
Weather patterns: Condensation and deposition are integral to weather phenomena, such as rain, snow, and fog formation. The heat released during these processes influences atmospheric temperature and circulation patterns.
-
Energy storage: Research is exploring the potential of utilizing exothermic phase transitions for energy storage applications. The heat released during phase changes can be captured and used later.
Distinguishing between Exothermic and Endothermic Phase Transitions
It's crucial to distinguish between exothermic and endothermic phase transitions. Remember this simple rule:
-
Exothermic: Energy is released (heat flows out of the system). The system's energy decreases. This happens when going from a higher energy state (gas) to a lower energy state (liquid or solid).
-
Endothermic: Energy is absorbed (heat flows into the system). The system's energy increases. This happens when going from a lower energy state (solid) to a higher energy state (liquid or gas).
Conclusion
Exothermic phase transitions are an essential part of the natural world and various technological processes. Understanding the underlying principles of these transitions, the factors influencing them, and their practical applications is crucial for a comprehensive understanding of matter and energy. By grasping the concept of heat flow during phase changes, we can better appreciate the diverse ways in which matter interacts with its environment and the energy transformations that occur. This knowledge is valuable across various scientific disciplines and engineering applications. From designing efficient refrigeration systems to predicting weather patterns, understanding exothermic phase transitions proves to be an invaluable tool.
Latest Posts
Latest Posts
-
Measurement Of The Amount Of Matter In An Object
Mar 20, 2025
-
Significant Figures Addition And Subtraction Practice
Mar 20, 2025
-
How To Calculate Ph At The Equivalence Point
Mar 20, 2025
-
Conversion From Rectangular To Spherical Coordinates
Mar 20, 2025
-
How Does A Magnifying Glass Work Physics
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Which Change Of Phase Is Exothermic . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
