Which Element Has Highest Ionization Energy
Muz Play
Mar 18, 2025 · 6 min read
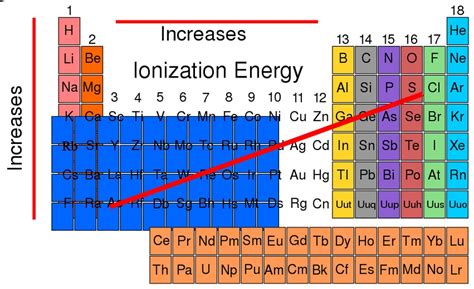
Table of Contents
Which Element Has the Highest Ionization Energy? Unveiling the Secrets of Atomic Structure
The quest to identify the element boasting the highest ionization energy takes us on a fascinating journey into the heart of atomic structure and the forces that govern electron behavior. Ionization energy, the minimum energy required to remove an electron from a gaseous atom or ion, is a fundamental property reflecting an atom's tenacity in holding onto its electrons. Understanding this property unlocks insights into chemical reactivity, bonding, and the periodic trends that shape the elements' behavior. This article will delve deep into the factors influencing ionization energy, explore the periodic trends, and ultimately reveal which element reigns supreme in this atomic tug-of-war.
Understanding Ionization Energy: A Closer Look
Before we embark on our search for the champion of ionization energy, let's solidify our understanding of the concept. Ionization energy, often measured in electronvolts (eV) or kilojoules per mole (kJ/mol), is not a single value but rather a series of values corresponding to successive removals of electrons. The first ionization energy (IE₁) refers to the energy required to remove the outermost electron, the second ionization energy (IE₂) to remove the next electron, and so on. Each subsequent ionization energy is progressively higher, as removing an electron from an increasingly positively charged ion requires more energy to overcome the stronger electrostatic attraction.
Several factors significantly influence an atom's ionization energy:
1. Nuclear Charge: The Stronger the Pull, the Higher the Energy
The positive charge of the nucleus exerts a powerful electrostatic attraction on the negatively charged electrons. A higher nuclear charge translates to a stronger pull, making it more difficult to remove an electron, thereby increasing ionization energy. This is a primary factor influencing the periodic trend of ionization energy.
2. Atomic Radius: Distance Matters
The distance between the nucleus and the outermost electrons, represented by the atomic radius, significantly impacts ionization energy. A smaller atomic radius means the outermost electrons are closer to the nucleus, experiencing a stronger electrostatic attraction. Consequently, removing an electron requires more energy, resulting in a higher ionization energy.
3. Shielding Effect: Inner Electrons' Protective Role
Inner electrons act as a shield, partially blocking the attractive force of the nucleus from reaching the outermost electrons. This shielding effect reduces the effective nuclear charge experienced by the valence electrons. The more inner electrons present, the greater the shielding, leading to a lower ionization energy.
4. Electron Configuration: Stability is Key
The stability of an electron configuration directly influences ionization energy. Elements with stable electron configurations, such as those with filled or half-filled subshells (like noble gases and elements with half-filled p or d subshells), exhibit higher ionization energies because removing an electron would disrupt this stability.
Periodic Trends in Ionization Energy: A Journey Across the Table
As we traverse the periodic table, ionization energy demonstrates clear trends:
1. Across a Period (Left to Right): The Steady Climb
Moving from left to right across a period, the nuclear charge increases while the shielding effect remains relatively constant due to the addition of electrons to the same principal energy level. This results in a stronger attraction between the nucleus and electrons, leading to a steady increase in ionization energy.
2. Down a Group (Top to Bottom): The Gradual Decrease
Descending a group, the atomic radius increases significantly due to the addition of new electron shells. The increased distance between the nucleus and valence electrons weakens the electrostatic attraction, resulting in a decrease in ionization energy. Additionally, the increased shielding effect from inner electrons further contributes to this decrease.
Exceptions to the Rules: The Intriguing Anomalies
While the general trends are quite predictable, exceptions exist. For example, the ionization energy of Boron is slightly lower than that of Beryllium, despite the increasing nuclear charge. This is attributed to the added electron residing in a higher energy p-orbital, experiencing less effective nuclear charge due to increased shielding. Similarly, Oxygen's ionization energy is slightly lower than that of Nitrogen. This is because pairing electrons in the same p-orbital leads to increased electron-electron repulsion, making it slightly easier to remove an electron from Oxygen.
The Contender for the Highest Ionization Energy: Helium's Reign
Based on the aforementioned factors, and considering the periodic trends, it is clear that elements with high nuclear charges, small atomic radii, minimal shielding, and stable electron configurations exhibit the highest ionization energies. Considering these criteria, the element with the highest first ionization energy is Helium (He).
Helium's exceptional ionization energy stems from its simple atomic structure: two protons in the nucleus and two electrons in the 1s orbital. This fully filled 1s subshell represents the epitome of electron configuration stability, making it extraordinarily difficult to remove an electron. The small atomic radius and strong nuclear charge further contribute to its extremely high ionization energy.
While Helium holds the title for the highest first ionization energy, it's important to note that subsequent ionization energies increase dramatically. Removing the second electron from He⁺ requires considerably more energy due to the increased positive charge of the ion and the strong attraction between the remaining electron and the nucleus.
Beyond Helium: Exploring High Ionization Energies
While Helium leads the pack in terms of first ionization energy, other elements exhibit exceptionally high ionization energies, particularly in their higher ionization states. Elements with high nuclear charges, such as those found in the later periods of the periodic table, demonstrate increasingly high ionization energies as more electrons are removed. However, these values become progressively challenging to measure experimentally due to the extremely high energies involved.
Understanding the factors influencing ionization energy and recognizing the periodic trends allows us to predict relative ionization energy values across the periodic table. While Helium claims the top spot for its first ionization energy, the values for subsequent ionization energies become increasingly higher for all elements, emphasizing the increasing difficulty in removing electrons from increasingly positive ions.
Applications of Ionization Energy: Real-World Relevance
The concept of ionization energy is not confined to theoretical discussions; it has significant real-world applications:
-
Spectroscopy: Ionization energies are crucial in understanding atomic spectra, allowing scientists to analyze the light emitted or absorbed by atoms and molecules, providing insights into their composition and structure.
-
Chemical Bonding: Ionization energy is central to understanding chemical bonding, as it helps predict the likelihood of an atom losing or gaining electrons to form ionic bonds.
-
Material Science: Ionization energy plays a role in understanding the properties of materials, particularly their conductivity and reactivity, influencing the design and development of new materials with specific characteristics.
Conclusion: A Deeper Understanding
The search for the element with the highest ionization energy has taken us on a comprehensive exploration of atomic structure, the factors influencing electron behavior, and the periodic trends that govern this fundamental property. Helium, with its exceptionally stable electron configuration, small atomic radius, and strong nuclear charge, reigns supreme in terms of first ionization energy. However, the concept extends far beyond this singular element, offering invaluable insights into the behavior of matter and finding broad applications in various scientific disciplines. By understanding ionization energy, we gain a deeper appreciation for the intricate forces shaping the atomic world and the remarkable properties of elements.
Latest Posts
Latest Posts
-
Find The Arclength Of The Curve
Mar 18, 2025
-
Which One Leaves The Solution Untouched
Mar 18, 2025
-
Which Statement Summarizes The Law Of Segregation
Mar 18, 2025
-
How Many Valence Electrons Does O3 Have
Mar 18, 2025
-
Why Do Solids Have A Definite Shape And Volume
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Which Element Has Highest Ionization Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
