Which Element Tends Not To React With Other Elements
Muz Play
Mar 18, 2025 · 5 min read
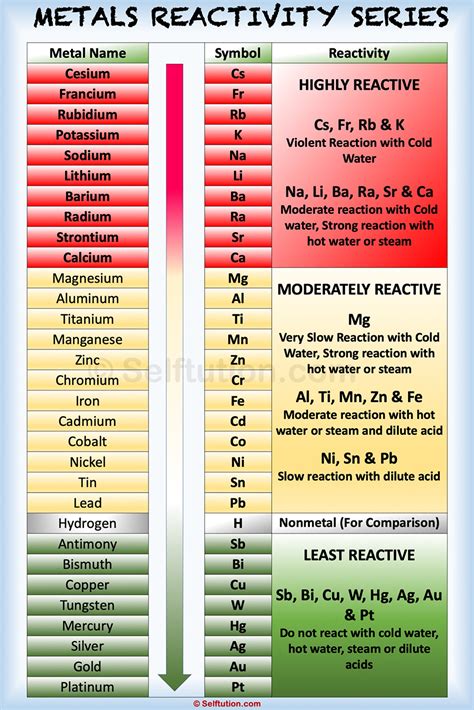
Table of Contents
Which Element Tends Not to React with Other Elements? Understanding Noble Gases and Chemical Inertness
The periodic table, a seemingly simple arrangement of elements, reveals a fascinating story of reactivity and stability. While many elements readily combine to form compounds, exhibiting a diverse range of chemical behaviors, some stand apart, showing a remarkable reluctance to engage in chemical reactions. This article delves into the world of chemical inertness, focusing on the elements that tend not to react with other elements: the noble gases. We will explore their unique electronic configurations, their historical discovery, and their surprising applications despite their inert nature.
The Noble Gases: A Family of Unreactive Elements
The noble gases, also known as inert gases, comprise Group 18 of the periodic table. This group includes helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), radon (Rn), and oganesson (Og). Their defining characteristic is their exceptional stability and minimal tendency to react with other elements. This inertness stems from their complete valence electron shells.
Understanding Valence Electrons and Chemical Bonding
Before delving into the specifics of noble gas inertness, it's crucial to understand the role of valence electrons. Valence electrons are the electrons in the outermost shell of an atom. These electrons are involved in chemical bonding, the process by which atoms interact and combine to form molecules or compounds. Atoms tend to react to achieve a stable electron configuration, often resembling the configuration of a noble gas, with a complete outer shell. This principle is often referred to as the octet rule (eight valence electrons), although exceptions exist, particularly for elements in the later periods of the periodic table.
The Complete Octet: The Key to Noble Gas Inertness
The noble gases possess a unique electronic configuration: their outermost electron shell is completely filled. For helium, this means two electrons (filling the 1s orbital), while for the other noble gases, it means eight electrons. This complete octet renders them exceptionally stable, with little tendency to gain, lose, or share electrons to form chemical bonds. They essentially have no "desire" to interact with other elements because they already possess the most stable electron arrangement possible.
A Historical Look at the Discovery of Noble Gases
The discovery of noble gases was a testament to scientific curiosity and the evolving understanding of matter. Unlike many elements known since antiquity, the noble gases remained elusive until the late 19th and early 20th centuries:
- Helium (He): First detected in the Sun's spectrum in 1868 by Pierre Janssen and Norman Lockyer before its terrestrial discovery.
- Argon (Ar): Isolated in 1894 by Lord Rayleigh and William Ramsay, initially identified by its unexpected density discrepancy in atmospheric nitrogen. This discovery opened the door to the recognition of an entire new group of elements.
- Neon (Ne), Krypton (Kr), and Xenon (Xe): Also isolated by Ramsay and his colleagues through fractional distillation of liquid air, starting in 1898.
- Radon (Rn): Discovered in 1900 by Friedrich Ernst Dorn as a radioactive decay product of radium.
- Oganesson (Og): A synthetically created element, first observed in 2002, extremely short-lived and radioactive.
The discovery of noble gases revolutionized our understanding of chemical reactivity and expanded the periodic table significantly. It highlighted the existence of elements that defied the conventional rules of chemical bonding.
Exceptions to the Rule: The Reactivity of Xenon and Other Noble Gases
While the noble gases are generally considered inert, the term "inert" is somewhat misleading. The heavier noble gases, particularly xenon, show a slight tendency to form compounds under specific conditions. This reactivity is attributed to the relatively large size of their atoms, which leads to weaker attraction of their valence electrons and hence increased polarizability. The increase in nuclear charge doesn't increase as rapidly with atomic radius as the shielding effect, meaning the outermost electrons are less strongly held by the nucleus.
Xenon Compounds: A Challenge to Inertness
Xenon forms a limited number of compounds, primarily with highly electronegative elements like fluorine and oxygen. Examples include xenon hexafluoride (XeF₆), xenon tetrafluoride (XeF₄), and xenon dioxide (XeO₂). These compounds require specific conditions, such as high pressures and temperatures or the use of strong oxidizing agents. This reactivity, however, remains far less significant than that observed in other elements.
The Reactivity of Other Noble Gases: Extremely Limited
While xenon demonstrates some reactivity, the other noble gases exhibit even less. Krypton can form a few compounds, but their stability is far lower than that of xenon compounds. Neon, argon, and helium are considered essentially unreactive under normal conditions. Radon, being radioactive and short-lived, has limited opportunities for chemical interactions. Oganesson's extreme radioactivity and short lifespan makes it unlikely that stable compounds will be discovered.
Applications of Noble Gases: Harnessing Inertness
Despite their inertness, noble gases have found various practical applications in diverse fields. Their lack of reactivity makes them ideal for situations requiring stability and lack of interference with other materials:
Helium (He): Balloons, MRI, and Cryogenics
Helium, the second lightest element, is widely used in applications requiring low density, such as weather balloons and blimps. Its inertness and ability to maintain a liquid state at extremely low temperatures make it essential in cryogenics and Magnetic Resonance Imaging (MRI) machines.
Neon (Ne), Argon (Ar), Krypton (Kr), and Xenon (Xe): Lighting, Welding, and Medicine
Neon, argon, krypton, and xenon are often used in lighting, owing to their characteristic colors when electrically excited. Argon is also used in welding to provide an inert atmosphere that protects the weld from oxidation. Xenon is used in some medical applications, including certain types of lasers used in eye surgery.
Radon (Rn): Radioactive Decay and Medical Applications
Radon is a radioactive gas, used in radiation therapy for cancer treatment, although its use is carefully controlled due to its radioactivity.
Conclusion: The Enduring Significance of Noble Gases
The noble gases, with their exceptional chemical inertness, stand out as a remarkable group on the periodic table. Their unique electronic configuration, leading to remarkable stability, is a cornerstone of chemical understanding. Although some heavier noble gases exhibit limited reactivity under extreme conditions, their general inertness makes them invaluable in various applications, from weather balloons to high-tech medical equipment. The ongoing exploration of their behavior continues to offer insights into the fundamental forces governing the behavior of matter. Their story highlights the interplay between fundamental properties of elements and their practical applications, underscoring the dynamic relationship between scientific discovery and technological advancement.
Latest Posts
Latest Posts
-
Delta G Of A Carbonyl Reduction
Mar 18, 2025
-
Difference Between A Strong And Weak Acid
Mar 18, 2025
-
What Are The Five Evaluation Criteria
Mar 18, 2025
-
What Aspects Of Politics Are Sociologists Generally Concerned With
Mar 18, 2025
-
Root Stems And Leaves Maintain Homeostasis
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Which Element Tends Not To React With Other Elements . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
