Which Group Has The Highest Ionization Energy
Muz Play
Mar 18, 2025 · 5 min read
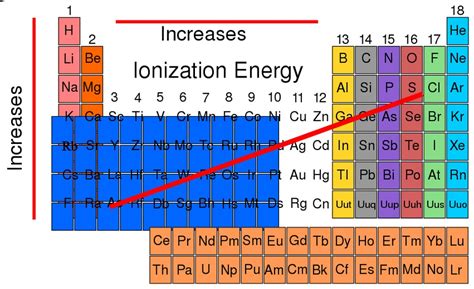
Table of Contents
Which Group Has the Highest Ionization Energy? Exploring Periodic Trends
Ionization energy, a fundamental concept in chemistry, dictates the energy required to remove an electron from an atom or ion in its gaseous phase. Understanding ionization energy trends across the periodic table is crucial for predicting chemical reactivity and behavior. While the straightforward answer is that Group 18 (noble gases) possesses the highest ionization energies, the reality is more nuanced and involves a deeper dive into atomic structure and electron configurations. This article will explore the reasons behind this trend, considering exceptions and providing a comprehensive understanding of the factors influencing ionization energy.
Understanding Ionization Energy: A Deeper Dive
Ionization energy isn't a single value; rather, it's a series of values, each representing the energy needed to remove successive electrons. The first ionization energy (IE₁) is the energy required to remove the outermost electron, the second ionization energy (IE₂) is the energy required to remove the second outermost electron, and so on. These values progressively increase because removing an electron leaves a positively charged ion, making it increasingly difficult to remove subsequent electrons due to the stronger electrostatic attraction between the nucleus and the remaining electrons.
Factors Affecting Ionization Energy
Several key factors influence the magnitude of ionization energy:
-
Nuclear Charge: A higher positive charge in the nucleus exerts a stronger attractive force on the electrons, increasing ionization energy. As you move across a period (left to right), the nuclear charge increases, leading to a higher ionization energy.
-
Atomic Radius: A smaller atomic radius means the outermost electrons are closer to the nucleus, experiencing a stronger attractive force and therefore higher ionization energy. Across a period, atomic radius decreases, contributing to the increase in ionization energy. Down a group, atomic radius increases, resulting in lower ionization energy.
-
Shielding Effect: Inner electrons shield the outer electrons from the full positive charge of the nucleus. This shielding effect reduces the effective nuclear charge experienced by the outermost electrons, decreasing ionization energy. The shielding effect is less effective across a period and more effective down a group.
-
Electron Configuration: Electrons in filled or half-filled subshells (s² and p⁶ configurations) are more stable than those in partially filled subshells. This increased stability requires more energy to remove electrons from these configurations, resulting in higher ionization energies.
Noble Gases: The Champions of High Ionization Energy
As mentioned earlier, Group 18, the noble gases (He, Ne, Ar, Kr, Xe, Rn), generally exhibit the highest first ionization energies in their respective periods. This is directly attributable to several factors working in concert:
-
Full Valence Shells: Noble gases possess a complete octet (eight valence electrons) or a duet (two valence electrons in the case of helium). This stable electron configuration makes them exceptionally unreactive and resistant to losing an electron. The strong electrostatic attraction between the nucleus and the filled valence shell significantly increases the ionization energy.
-
High Effective Nuclear Charge: While shielding effects exist, the strong nuclear charge in noble gases, combined with the compact size of their atoms, results in a high effective nuclear charge experienced by the valence electrons. This enhances the electrostatic attraction, contributing to the high ionization energy.
-
Minimal Electron-Electron Repulsion: The filled valence shells minimize electron-electron repulsion, further enhancing the stability of the noble gas configuration and increasing the energy required to remove an electron.
Exceptions and Anomalies: Understanding the Nuances
While noble gases generally exhibit the highest ionization energies, there are some exceptions and nuances to consider:
-
Helium (He): Helium, with only two electrons, forms a stable duet configuration, making its first ionization energy exceptionally high compared to other elements in its period.
-
Variations within Groups: The increase in ionization energy down a group is not strictly linear. Relativistic effects, particularly significant for heavier elements, can cause slight deviations from the general trend. These effects influence the electron shielding and the attraction between the nucleus and the electrons.
-
Comparing Ionization Energies: Direct comparison of ionization energies requires careful consideration of the various factors discussed. The difference between the first and subsequent ionization energies of the same element can be enormous, highlighting the increasing difficulty of removing electrons as the atom becomes more positively charged.
The Importance of Ionization Energy in Chemistry
The concept of ionization energy is critical to understanding many aspects of chemistry, including:
-
Chemical Reactivity: Elements with low ionization energies tend to be highly reactive, readily losing electrons to form positive ions (cations). Conversely, elements with high ionization energies are less reactive, as they strongly resist electron loss.
-
Bond Formation: Ionization energy plays a vital role in ionic bond formation. The transfer of electrons from an element with low ionization energy to an element with high electron affinity leads to the formation of ionic compounds.
-
Spectroscopy: Analyzing the ionization energies of atoms and ions provides insights into their electronic structure and energy levels, a key application in atomic spectroscopy.
Conclusion: A Comprehensive Understanding
While Group 18 (noble gases) generally possesses the highest first ionization energies, a comprehensive understanding requires acknowledging the intricate interplay between nuclear charge, atomic radius, shielding effects, and electron configuration. These factors dictate the energy required to remove an electron, influencing chemical reactivity and numerous other properties of elements. The variations and exceptions within the general trends highlight the complexity of atomic interactions and the necessity of a nuanced approach when analyzing ionization energy data. By considering these factors, we gain a far deeper understanding of the fundamental principles governing the behavior of matter at the atomic level.
Latest Posts
Latest Posts
-
Is Melting Point Physical Or Chemical Property
Mar 18, 2025
-
Find The Arclength Of The Curve
Mar 18, 2025
-
Which One Leaves The Solution Untouched
Mar 18, 2025
-
Which Statement Summarizes The Law Of Segregation
Mar 18, 2025
-
How Many Valence Electrons Does O3 Have
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Which Group Has The Highest Ionization Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
