Which Intermolecular Force Is The Strongest
Muz Play
Mar 18, 2025 · 6 min read
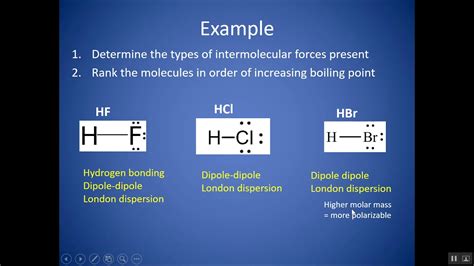
Table of Contents
Which Intermolecular Force is the Strongest? A Deep Dive into Molecular Interactions
Understanding intermolecular forces is crucial for comprehending the properties of matter, from the boiling point of water to the structure of proteins. While the strength of these forces varies considerably, a common question arises: which intermolecular force is the strongest? The answer isn't as straightforward as it might seem, as the strength of these forces is context-dependent and relative to the molecules involved. However, we can analyze the major players and understand their relative strengths to gain a clearer picture.
The Contenders: A Review of Major Intermolecular Forces
Before diving into comparisons, let's briefly review the key players in the intermolecular force arena:
1. Ion-Dipole Forces: The Strongest of the Intermolecular Forces (Generally)
Ion-dipole forces occur between an ion (either a cation or an anion) and a polar molecule. The charged ion strongly attracts the oppositely charged end of the polar molecule. These are generally considered the strongest intermolecular forces, surpassing even hydrogen bonds in certain scenarios. The strength of the interaction is directly proportional to the charge of the ion and the dipole moment of the polar molecule. Think of the interaction between sodium ions (Na⁺) and water molecules (H₂O) in a saltwater solution – the strong attraction between the positively charged sodium ion and the partially negative oxygen atom in water is a prime example of a powerful ion-dipole force.
Factors Influencing Ion-Dipole Strength:
- Ionic Charge: Higher ionic charge leads to stronger attraction.
- Dipole Moment: A larger dipole moment in the polar molecule results in stronger interaction.
- Distance: The force weakens rapidly with increasing distance.
2. Hydrogen Bonds: Specialized Dipole-Dipole Interactions
Hydrogen bonds are a special type of dipole-dipole interaction that occurs when a hydrogen atom is bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine) and is attracted to another electronegative atom in a nearby molecule. The high electronegativity difference creates a strong partial positive charge on the hydrogen and a strong partial negative charge on the electronegative atom. This results in a relatively strong attraction. Water's unique properties, including its high boiling point and surface tension, are a direct result of its extensive hydrogen bonding network.
Key Characteristics of Hydrogen Bonds:
- Stronger than typical dipole-dipole forces: Due to the large electronegativity difference and relatively small size of hydrogen.
- Directionality: Hydrogen bonds have a specific directionality, influencing the structure and properties of molecules.
- Cooperative effects: Hydrogen bonds can strengthen each other through cooperative effects.
3. Dipole-Dipole Forces: Attractions Between Polar Molecules
Dipole-dipole forces are attractive forces between polar molecules. Polar molecules have a permanent dipole moment due to an uneven distribution of electrons. The partially positive end of one molecule attracts the partially negative end of another, leading to an attractive force. While weaker than ion-dipole forces and hydrogen bonds, dipole-dipole forces still significantly influence the properties of polar substances.
Factors Affecting Dipole-Dipole Strength:
- Dipole Moment: Larger dipole moments result in stronger interactions.
- Molecular Shape: Molecular shape influences the effectiveness of dipole-dipole interactions.
4. London Dispersion Forces (LDFs): Weakest but Ubiquitous
London dispersion forces, also known as van der Waals forces, are the weakest type of intermolecular force. They arise from temporary, instantaneous fluctuations in electron distribution around atoms and molecules. These fluctuations create temporary dipoles, which induce dipoles in neighboring molecules, leading to weak attractive forces. While individually weak, LDFs are present in all molecules, and their cumulative effect can be substantial, especially in large molecules with many electrons.
Factors Influencing LDF Strength:
- Molecular Size and Shape: Larger molecules with larger surface areas have stronger LDFs due to increased polarizability.
- Molecular Polarizability: The ease with which electron clouds can be distorted influences LDF strength.
The Strength Comparison: A Nuance-Rich Answer
Declaring a single "strongest" intermolecular force is an oversimplification. The strength of an intermolecular force depends heavily on the specific molecules involved and the context.
-
Ion-dipole forces generally reign supreme: If an ion is present, the interaction with a polar molecule will typically be the strongest. The magnitude of the ionic charge and the dipole moment of the polar molecule dictates the strength.
-
Hydrogen bonds are exceptionally strong for neutral molecules: Amongst forces between neutral molecules, hydrogen bonds often dominate. Their strength arises from the exceptionally large electronegativity difference and the unique characteristics of the hydrogen atom.
-
Dipole-dipole forces are intermediate in strength: They are stronger than LDFs but weaker than ion-dipole forces and hydrogen bonds.
-
London Dispersion forces are the weakest: However, their cumulative effect can be substantial in large molecules or in situations where other forces are absent.
Context Matters: Examples Highlighting the Nuances
Let's examine specific examples to illustrate the nuances of comparing intermolecular forces:
Example 1: NaCl in Water
When sodium chloride (NaCl) dissolves in water, the strong ion-dipole forces between Na⁺ ions and water molecules, and Cl⁻ ions and water molecules, overcome the strong ionic bonds within the NaCl crystal lattice. This demonstrates that ion-dipole forces can be stronger than ionic bonds in certain contexts.
Example 2: Water vs. Methane
Water (H₂O) has a significantly higher boiling point than methane (CH₄) despite both having similar molecular weights. This is attributed to the strong hydrogen bonding in water, which is far stronger than the weak London dispersion forces present in nonpolar methane.
Example 3: Large Hydrocarbons
Large hydrocarbons, like octane (C₈H₁₈), primarily experience London dispersion forces. Although individually weak, the cumulative effect of numerous LDFs across the large molecule contributes to a relatively high boiling point compared to smaller molecules with weaker LDFs.
Factors Beyond the Primary Forces: Influencing Intermolecular Interactions
Beyond the main intermolecular forces discussed, several additional factors play a crucial role:
-
Molecular Shape and Geometry: The spatial arrangement of atoms within a molecule significantly affects the strength and efficiency of intermolecular interactions. A linear molecule, for instance, may exhibit stronger dipole-dipole interactions than a bent molecule with the same dipole moment.
-
Polarizability: This refers to the ease with which the electron cloud of an atom or molecule can be distorted. Higher polarizability leads to stronger London dispersion forces.
-
Temperature: Higher temperatures provide molecules with greater kinetic energy, which can counteract the attractive intermolecular forces. This is why substances transition from solid to liquid to gas as temperature increases.
Conclusion: A Holistic Perspective on Intermolecular Force Strength
While ion-dipole forces generally represent the strongest intermolecular interactions when considering ions, the strength of intermolecular forces is highly dependent on the specific molecules involved and the interplay of different factors. Hydrogen bonding emerges as a dominant force among interactions between neutral molecules, with dipole-dipole forces holding an intermediate position, and London dispersion forces representing the weakest but ubiquitous type. A comprehensive understanding requires acknowledging the nuances of each force, the context in which they operate, and the impact of factors such as molecular shape, polarizability, and temperature. Understanding these subtleties is essential for a thorough grasp of the physical and chemical properties of matter.
Latest Posts
Latest Posts
-
Find The Arclength Of The Curve
Mar 18, 2025
-
Which One Leaves The Solution Untouched
Mar 18, 2025
-
Which Statement Summarizes The Law Of Segregation
Mar 18, 2025
-
How Many Valence Electrons Does O3 Have
Mar 18, 2025
-
Why Do Solids Have A Definite Shape And Volume
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Which Intermolecular Force Is The Strongest . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
