Which Of The Following Is An Oxidation Reduction Reaction
Muz Play
Mar 20, 2025 · 6 min read
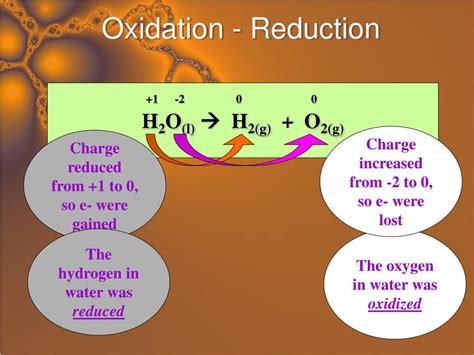
Table of Contents
Which of the Following is an Oxidation-Reduction Reaction? A Deep Dive into Redox Chemistry
Oxidation-reduction reactions, commonly known as redox reactions, are fundamental chemical processes that involve the transfer of electrons between species. Understanding redox reactions is crucial in various fields, from biology and environmental science to materials science and industrial chemistry. This article will delve into the intricacies of redox reactions, providing a comprehensive guide to identifying them and understanding their significance. We will explore various examples and explain how to determine if a given reaction is a redox reaction.
Defining Oxidation and Reduction
Before we delve into identifying redox reactions, let's firmly establish the definitions of oxidation and reduction. These two processes are always coupled; one cannot occur without the other.
Oxidation: This refers to the loss of electrons by a chemical species. The species that loses electrons is said to be oxidized. Oxidation often involves an increase in oxidation state (a number assigned to an atom reflecting its apparent charge).
Reduction: This refers to the gain of electrons by a chemical species. The species that gains electrons is said to be reduced. Reduction often involves a decrease in oxidation state.
The mnemonic device OIL RIG is often used to remember these definitions: Oxidation Is Loss (of electrons), Reduction Is Gain (of electrons).
Identifying Redox Reactions: Key Indicators
Several key indicators can help you determine if a given chemical reaction is a redox reaction. Let's explore these:
1. Changes in Oxidation States
The most reliable method for identifying a redox reaction is to track the changes in oxidation states of the elements involved. If the oxidation state of at least one element increases (oxidation) and the oxidation state of at least one other element decreases (reduction), then the reaction is a redox reaction.
Example: Consider the reaction between iron(II) ions and permanganate ions in an acidic solution:
5Fe²⁺(aq) + MnO₄⁻(aq) + 8H⁺(aq) → 5Fe³⁺(aq) + Mn²⁺(aq) + 4H₂O(l)
Here:
- Iron (Fe) goes from an oxidation state of +2 to +3 (oxidation – loss of an electron).
- Manganese (Mn) goes from an oxidation state of +7 to +2 (reduction – gain of electrons).
Since both oxidation and reduction occur simultaneously, this is a redox reaction.
2. Presence of Oxidizing and Reducing Agents
Redox reactions always involve two key players:
-
Oxidizing agent: A species that accepts electrons, causing another species to be oxidized. The oxidizing agent itself is reduced in the process. Common oxidizing agents include oxygen (O₂), halogens (F₂, Cl₂, Br₂, I₂), and permanganate ions (MnO₄⁻).
-
Reducing agent: A species that donates electrons, causing another species to be reduced. The reducing agent itself is oxidized in the process. Common reducing agents include metals (like alkali metals and alkaline earth metals), and some nonmetals (like hydrogen).
In the previous example, MnO₄⁻ acts as the oxidizing agent (it's reduced), and Fe²⁺ acts as the reducing agent (it's oxidized).
3. Transfer of Electrons
While changes in oxidation states are the most definitive indicator, you can also look for the direct transfer of electrons in the balanced chemical equation. If electrons appear explicitly as reactants or products, it's a clear sign of a redox reaction.
Example: The reaction between sodium and chlorine:
2Na(s) + Cl₂(g) → 2NaCl(s)
This can be broken down into half-reactions:
2Na(s) → 2Na⁺(g) + 2e⁻(Oxidation: Sodium loses electrons)Cl₂(g) + 2e⁻ → 2Cl⁻(g)(Reduction: Chlorine gains electrons)
The explicit transfer of electrons confirms this reaction as a redox reaction.
Common Types of Redox Reactions
Redox reactions encompass a broad range of chemical processes. Here are some common types:
1. Combustion Reactions
Combustion involves the rapid reaction of a substance with oxygen, producing heat and light. The substance being oxidized (fuel) loses electrons to oxygen (oxidizing agent), which is reduced.
Example: The combustion of methane:
CH₄(g) + 2O₂(g) → CO₂(g) + 2H₂O(l)
Carbon in methane is oxidized (its oxidation state increases), while oxygen is reduced (its oxidation state decreases).
2. Corrosion and Rusting
Corrosion, like the rusting of iron, is a redox process where a metal reacts with its environment to form an oxide. The metal is oxidized, while oxygen or another oxidizing agent is reduced.
Example: Rusting of iron:
4Fe(s) + 3O₂(g) → 2Fe₂O₃(s)
Iron is oxidized, and oxygen is reduced.
3. Single Displacement Reactions (or Replacement Reactions)
In these reactions, one element replaces another in a compound. The displaced element is oxidized, and the replacing element is reduced.
Example: Reaction of zinc with copper(II) sulfate:
Zn(s) + CuSO₄(aq) → ZnSO₄(aq) + Cu(s)
Zinc is oxidized, and copper is reduced.
4. Disproportionation Reactions
These reactions involve a single element undergoing both oxidation and reduction simultaneously. A single species is both oxidized and reduced.
Example: Reaction of hydrogen peroxide:
2H₂O₂(aq) → 2H₂O(l) + O₂(g)
In this reaction, oxygen in H₂O₂ is both oxidized (in O₂) and reduced (in H₂O).
Examples of Reactions: Redox or Not?
Let's analyze several reactions to determine if they are redox reactions:
1. 2HCl(aq) + Ca(OH)₂(aq) → CaCl₂(aq) + 2H₂O(l)
This is an acid-base neutralization reaction, not a redox reaction. There are no changes in oxidation states.
2. 2Mg(s) + O₂(g) → 2MgO(s)
This is a redox reaction. Magnesium is oxidized (loses electrons), and oxygen is reduced (gains electrons).
3. AgNO₃(aq) + NaCl(aq) → AgCl(s) + NaNO₃(aq)
This is a precipitation reaction, not a redox reaction. There are no changes in oxidation states.
4. Cl₂(g) + 2KI(aq) → 2KCl(aq) + I₂(s)
This is a redox reaction. Chlorine is reduced (gains electrons), and iodine is oxidized (loses electrons).
5. Zn(s) + 2HCl(aq) → ZnCl₂(aq) + H₂(g)
This is a redox reaction. Zinc is oxidized (loses electrons), and hydrogen is reduced (gains electrons).
6. Fe₂O₃(s) + 3CO(g) → 2Fe(s) + 3CO₂(g)
This is a redox reaction. Iron is reduced (gains electrons), and carbon is oxidized (loses electrons). This is a common metallurgical process for iron extraction.
Applications of Redox Reactions
Redox reactions are ubiquitous in nature and technology. Some key applications include:
-
Batteries: Batteries rely on redox reactions to generate electrical energy. The flow of electrons from the oxidation half-reaction to the reduction half-reaction creates an electrical current.
-
Corrosion prevention: Understanding redox reactions is crucial for developing methods to prevent corrosion of metals, which causes significant economic damage.
-
Electroplating: Electroplating involves using redox reactions to deposit a thin layer of metal onto a surface.
-
Metallurgy: Extraction of metals from their ores often involves redox reactions.
-
Biological processes: Many crucial biological processes, including respiration and photosynthesis, are redox reactions. Cellular respiration involves the oxidation of glucose and the reduction of oxygen to produce energy. Photosynthesis is the reverse process, using light energy to reduce carbon dioxide and oxidize water.
Conclusion
Identifying redox reactions requires a thorough understanding of oxidation and reduction processes. By analyzing changes in oxidation states, identifying oxidizing and reducing agents, or observing the direct transfer of electrons, you can confidently determine whether a given chemical reaction is a redox reaction. The wide-ranging applications of redox reactions highlight their importance across numerous scientific and technological disciplines. Mastering the concepts of redox chemistry is essential for anyone pursuing studies or careers in related fields.
Latest Posts
Latest Posts
-
The Correct Order Of Events In Aerobic Respiration Is
Mar 21, 2025
-
Does Multiplicity Have Anything To Do With Generalized Eigenvectors
Mar 21, 2025
-
How To Do Data Transformations Statistics In Excel
Mar 21, 2025
-
Find All Real Zeros Of The Function
Mar 21, 2025
-
Does Electronegativity Increase Down A Group
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Is An Oxidation Reduction Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
