Which Phase Change Is An Endothermic Change
Muz Play
Mar 22, 2025 · 6 min read
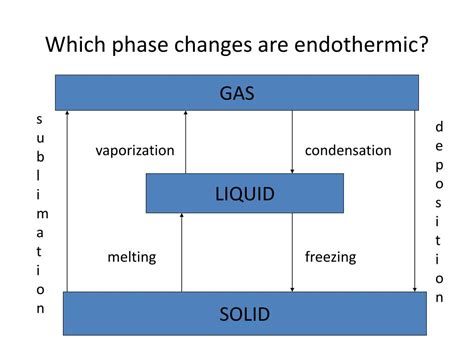
Table of Contents
Which Phase Change is an Endothermic Change? Understanding Energy Transfer in Matter
Phase transitions, the changes in the physical state of matter, are fundamental processes governed by the transfer of energy. Understanding whether a phase change is endothermic (absorbing energy) or exothermic (releasing energy) is crucial in various fields, from chemistry and physics to meteorology and materials science. This comprehensive article delves into the fascinating world of phase changes, focusing specifically on which transitions are endothermic, explaining the underlying principles, and providing real-world examples.
Understanding Endothermic Processes
Before diving into specific phase changes, let's establish a clear understanding of what constitutes an endothermic process. An endothermic process is any process that absorbs energy from its surroundings. This absorbed energy increases the internal energy of the system, leading to a change in its physical or chemical state. The energy absorbed can manifest in various forms, including heat, light, or electrical energy. In the context of phase changes, the energy absorbed manifests as heat, increasing the kinetic energy of the particles involved.
Think of it like this: If you're adding energy (heat) to a system and causing a change, it's an endothermic process. Conversely, if the system is releasing energy (heat) during a change, it's exothermic.
Phase Changes and Their Energy Requirements
Matter exists in various phases, most commonly solid, liquid, and gas (though plasma and Bose-Einstein condensates are other fascinating states). Transitions between these phases involve changes in the arrangement and interactions of particles. The energy required for these transitions dictates whether they are endothermic or exothermic.
Here’s a breakdown:
1. Melting (Solid to Liquid): An Endothermic Change
Melting is the process where a solid substance transforms into a liquid. For this to occur, sufficient energy must be supplied to overcome the strong intermolecular forces holding the particles in a fixed lattice structure in the solid state. This energy is absorbed as heat, causing the particles to gain kinetic energy and move more freely, breaking away from the rigid structure and transitioning to the liquid phase. Therefore, melting is an endothermic process.
Example: Ice melting into water. The ice absorbs heat from the surroundings, resulting in a temperature increase and a phase change from solid (ice) to liquid (water).
2. Vaporization (Liquid to Gas): An Endothermic Change
Vaporization encompasses two related processes: evaporation and boiling. Both involve the transition from a liquid to a gaseous state. To vaporize, liquid particles require sufficient energy to overcome the intermolecular forces holding them together in the liquid phase. This energy is absorbed from the surroundings, increasing the kinetic energy of the particles and allowing them to escape into the gaseous phase. Thus, vaporization is an endothermic process.
-
Evaporation: This occurs at the surface of a liquid at temperatures below the boiling point. Faster-moving particles with higher kinetic energy escape the liquid's surface.
-
Boiling: This occurs throughout the liquid at the boiling point, where the vapor pressure equals the atmospheric pressure. Bubbles of vapor form and rise to the surface.
Example: Water boiling in a kettle. The water absorbs heat from the kettle's element, reaching its boiling point and transitioning from liquid to gaseous water vapor.
3. Sublimation (Solid to Gas): An Endothermic Change
Sublimation is a less common but equally fascinating phase transition where a solid directly changes to a gas without passing through the liquid phase. Like melting and vaporization, sublimation requires energy input to overcome the intermolecular forces holding the particles in the solid state and allow them to escape into the gaseous phase. Therefore, sublimation is also an endothermic process.
Example: Dry ice (solid carbon dioxide) sublimating into carbon dioxide gas. The dry ice absorbs heat from its surroundings, directly transforming from a solid to a gas. This is why dry ice appears to "vanish" without leaving a liquid residue.
Exothermic Phase Changes: A Contrast
To fully understand endothermic phase changes, it's helpful to contrast them with exothermic phase changes. Exothermic processes release energy to the surroundings. In the context of phase transitions, this means that energy is given off during the change.
Here are the exothermic counterparts of the endothermic changes discussed above:
1. Freezing (Liquid to Solid): An Exothermic Change
Freezing is the opposite of melting. As a liquid cools, its particles lose kinetic energy, and the intermolecular forces become strong enough to hold the particles in a fixed lattice structure. This process releases energy in the form of heat to the surroundings. Therefore, freezing is an exothermic process.
Example: Water freezing into ice. As the water cools, it releases heat to its surroundings, resulting in a decrease in temperature and a phase change from liquid (water) to solid (ice).
2. Condensation (Gas to Liquid): An Exothermic Change
Condensation is the opposite of vaporization. As a gas cools, its particles lose kinetic energy, and the intermolecular forces become significant enough to pull the particles closer together, forming a liquid. This process releases energy in the form of heat to the surroundings. Therefore, condensation is an exothermic process.
Example: Water vapor condensing on a cold surface. The water vapor releases heat to the cold surface, cooling down and condensing into liquid water droplets.
3. Deposition (Gas to Solid): An Exothermic Change
Deposition is the opposite of sublimation. As a gas cools, its particles lose kinetic energy, and the intermolecular forces pull the particles directly into a solid structure without passing through the liquid phase. This process releases energy in the form of heat to the surroundings. Therefore, deposition is an exothermic process.
Example: Frost forming on a cold surface. Water vapor in the air directly transitions to ice crystals on a cold surface, releasing heat in the process.
Real-World Applications and Significance
Understanding endothermic and exothermic phase changes is critical in numerous applications:
-
Refrigeration and Air Conditioning: These systems rely on the endothermic vaporization of refrigerants to absorb heat from the surroundings, cooling the air.
-
Meteorology: The processes of evaporation, condensation, and sublimation play pivotal roles in weather patterns, cloud formation, and precipitation.
-
Industrial Processes: Many industrial processes involve phase changes, such as distillation, crystallization, and freeze-drying, and understanding the energy requirements is crucial for optimization and efficiency.
-
Materials Science: The study of phase transitions is essential for designing and developing new materials with specific properties, like alloys and polymers.
-
Cooking: Melting butter, boiling water, or freezing ice cream all involve phase changes that are vital to the cooking process.
Conclusion: Endothermic Phase Changes as Energy Absorbers
In conclusion, melting, vaporization, and sublimation are the key endothermic phase changes. They all require an input of energy to overcome the intermolecular forces holding particles together in a less energetic state, leading to the transition to a more energetic state. This absorbed energy is crucial for a multitude of natural phenomena and technological applications. By understanding the energy dynamics of phase changes, we can better comprehend and manipulate the behavior of matter and harness its properties for various purposes.
Latest Posts
Latest Posts
-
How Many Types Of Speech Are There
Mar 22, 2025
-
Relation Between Linear Velocity And Angular Velocity
Mar 22, 2025
-
Draw The Ether With The Common Name Phenyl Propyl Ether
Mar 22, 2025
-
Where Does Dna Replication Occur In Eukaryotic Cells
Mar 22, 2025
-
Which State Of Matter Has Definite Shape And Definite Volume
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about Which Phase Change Is An Endothermic Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
