Draw The Ether With The Common Name Phenyl Propyl Ether
Muz Play
Mar 22, 2025 · 6 min read
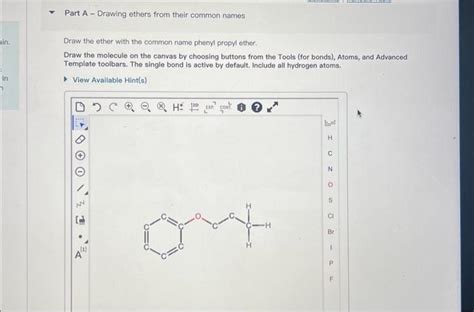
Table of Contents
Drawing the Ether with the Common Name Phenyl Propyl Ether: A Comprehensive Guide
Phenyl propyl ether, also known as 1-phenoxypropane, is an aromatic ether with a relatively simple structure. Understanding how to draw its structure is crucial for anyone studying organic chemistry, particularly those focusing on ether nomenclature and properties. This comprehensive guide will walk you through the process step-by-step, exploring the underlying principles of organic chemistry and providing practical tips for accurate representation.
Understanding the Nomenclature: Breaking Down Phenyl Propyl Ether
Before we delve into the drawing process, let's break down the name "phenyl propyl ether" to understand its structural implications. The name provides vital clues about the constituent parts of the molecule:
-
Ether: This tells us the molecule belongs to the ether functional group family, characterized by an oxygen atom bonded to two alkyl or aryl groups (R-O-R').
-
Propyl: This indicates a three-carbon alkyl chain (CH3CH2CH3).
-
Phenyl: This signifies a phenyl group, a benzene ring (C6H5).
Therefore, the name "phenyl propyl ether" indicates an oxygen atom bridging a phenyl group and a propyl group.
Step-by-Step Drawing Process: From Name to Structure
Let's now translate this understanding into a visual representation. We'll follow a methodical approach to ensure accuracy:
Step 1: Drawing the Core Structure - The Ether Linkage
Begin by drawing the central oxygen atom (O) representing the ether linkage. This oxygen atom will serve as the connecting point for both the phenyl and propyl groups.
O
Step 2: Adding the Propyl Group
Next, attach a three-carbon propyl chain to one side of the oxygen atom. Remember that a propyl group is a straight chain of three carbons. You can represent the carbons with their associated hydrogens, or use a condensed structure for brevity.
O
/
CH3CH2CH3
Step 3: Attaching the Phenyl Group
The final step involves attaching the phenyl group (benzene ring) to the other side of the oxygen atom. Remember that the phenyl group is a six-carbon ring with alternating single and double bonds. It is essential to depict the delocalized pi electrons appropriately (often represented as a circle within the ring) to accurately represent benzene's aromatic character.
CH3CH2CH3
|
O
/
C6H5 (or a benzene ring)
Step 4: Complete Structure of Phenyl Propyl Ether
The completed structure should show the oxygen atom bonded to both the propyl group and the phenyl group. This is the complete representation of phenyl propyl ether. You may represent the hydrogens explicitly or use a skeletal/condensed formula for convenience depending on context.
Alternative Representations: Condensed and Skeletal Structures
While the detailed structure shows every atom and bond, condensed and skeletal structures offer more concise representations:
Condensed Structure: C6H5OCH2CH2CH3
Skeletal Structure: This structure omits carbons and hydrogens, focusing solely on the bonds and heteroatoms. The skeletal structure would represent the phenyl group as a hexagon and the propyl group as a chain of three vertices. The oxygen atom would be explicitly shown connecting both groups.
Exploring the Properties of Phenyl Propyl Ether: Structure-Property Relationship
The structure of phenyl propyl ether directly influences its physical and chemical properties:
-
Aromatic Nature: The presence of the phenyl group imparts aromatic character, contributing to relatively high stability and specific reactivity patterns. Aromatic compounds often exhibit distinct UV-Vis absorption spectra and undergo electrophilic aromatic substitution reactions.
-
Ether Functionality: The ether linkage (-O-) influences the polarity of the molecule. Although less polar than alcohols, ethers still exhibit some dipole-dipole interactions, affecting their boiling points and solubility. Phenyl propyl ether's solubility in water is likely limited due to the significant non-polar phenyl group.
-
Boiling Point: Phenyl propyl ether's boiling point will be higher than similarly sized alkanes because of the dipole-dipole interactions and stronger dispersion forces stemming from its increased molecular size and polarity compared to comparable alkanes.
-
Reactivity: The ether linkage itself is relatively unreactive, but the presence of the aromatic ring introduces possibilities for electrophilic aromatic substitution reactions. The propyl chain may undergo some typical reactions of alkyl chains (e.g., oxidation).
-
Applications: The specific applications of phenyl propyl ether would depend on its reactivity and other properties. It might find use as a solvent, an intermediate in the synthesis of other compounds, or possibly in specialized applications that leverage its properties.
Deeper Dive into Organic Chemistry Concepts: Understanding the Ether Functional Group
A thorough understanding of the ether functional group is essential for grasping the properties and reactivity of phenyl propyl ether.
-
Bonding: The oxygen atom in the ether linkage has two lone pairs of electrons, leading to a bent molecular geometry. This geometry impacts the polarity of the molecule.
-
Polarity: Ethers exhibit dipole-dipole interactions due to the polar C-O bonds, although they are less polar than alcohols because they lack the highly polar O-H bond.
-
Solubility: The solubility of ethers depends on the size and polarity of the alkyl or aryl groups attached to the oxygen. Larger, non-polar groups reduce solubility in water, while smaller, polar groups increase it.
-
Reactivity: Ethers are generally relatively unreactive compared to other oxygen-containing functional groups, such as alcohols and carboxylic acids. However, strong acids can cleave the ether linkage under specific conditions.
-
Preparation of Ethers: Ethers can be prepared through various methods, including the Williamson ether synthesis (reaction of an alkoxide with an alkyl halide). The synthesis of phenyl propyl ether may involve a similar approach.
Advanced Topics: Spectroscopy and Identification of Phenyl Propyl Ether
Identifying and characterizing phenyl propyl ether would involve spectroscopic techniques:
-
Nuclear Magnetic Resonance (NMR) Spectroscopy: ¹H NMR and ¹³C NMR would reveal detailed structural information, including the chemical shifts of protons and carbons in different environments within the molecule.
-
Infrared (IR) Spectroscopy: The IR spectrum would show characteristic absorption bands for the C-O-C stretch (around 1250-1000 cm⁻¹), aromatic C-H stretches, and other functional groups.
-
Mass Spectrometry (MS): MS would reveal the molecular weight and fragmentation pattern of the molecule, providing further evidence for the structure.
Conclusion: Mastering the Art of Drawing and Understanding Organic Molecules
Drawing the structure of phenyl propyl ether is a fundamental skill in organic chemistry. By understanding the nomenclature, applying step-by-step drawing procedures, and exploring related organic chemistry concepts, you will gain a solid grasp of this molecule's structure and its properties. This knowledge provides a strong foundation for understanding more complex organic molecules and their behaviours. Remember to utilize various representations (detailed, condensed, skeletal) to facilitate understanding and communication. Furthermore, familiarizing yourself with spectroscopy techniques will allow you to identify and characterise this molecule effectively. Through practice and a strong understanding of the underlying principles, you can master the art of drawing and interpreting organic molecular structures.
Latest Posts
Latest Posts
-
Hydrogen Bonds Can Be Broken By
Mar 23, 2025
-
What Are 3 Shapes Of Bacteria
Mar 23, 2025
-
How Does Pressure Affect The Equilibrium
Mar 23, 2025
-
How To Find A Vector Perpendicular
Mar 23, 2025
-
How Did Catholics Respond To The Protestant Reformation
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Draw The Ether With The Common Name Phenyl Propyl Ether . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
