Which Place On An Enzyme Binds A Substrate
Muz Play
Mar 29, 2025 · 6 min read
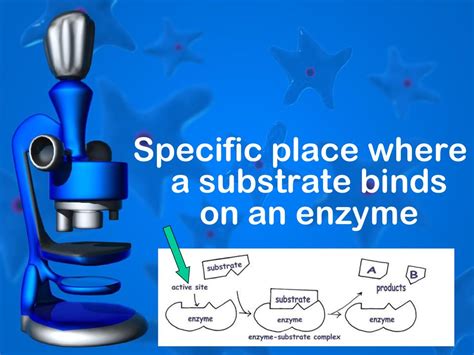
Table of Contents
Which Place on an Enzyme Binds a Substrate? Understanding the Active Site and Beyond
Enzymes are biological catalysts, remarkable molecules that accelerate the rate of virtually all chemical reactions within cells. Their power lies in their ability to specifically bind substrates, the molecules they act upon, at a precise location. This binding event is the crucial first step in enzymatic catalysis, initiating a cascade of events that ultimately lead to product formation. Understanding exactly where on an enzyme this binding occurs is fundamental to comprehending enzyme function, regulation, and design.
The Active Site: The Heart of Enzyme-Substrate Interaction
The primary site of substrate binding on an enzyme is the active site. This is a three-dimensional cleft or groove on the enzyme's surface, formed by amino acid residues from different parts of the polypeptide chain. The active site is not just a simple pocket; it's a highly specialized microenvironment meticulously sculpted by evolution to accommodate the substrate(s) with exquisite specificity and efficiency.
Key Features of the Active Site:
- Specificity: The active site possesses a unique shape and chemical environment that complements the substrate's structure. This ensures that the enzyme binds only its specific substrate(s), preventing unwanted side reactions and maintaining cellular order. This specificity is often compared to a "lock and key" model, though the more accurate "induced fit" model, discussed later, is now widely accepted.
- Binding Interactions: The substrate is held within the active site through a variety of non-covalent interactions, including hydrogen bonds, ionic bonds, hydrophobic interactions, and van der Waals forces. These interactions are relatively weak individually, but their cumulative effect ensures strong and specific substrate binding.
- Catalytic Residues: The active site contains specific amino acid residues, termed catalytic residues, that directly participate in the chemical transformation of the substrate. These residues may facilitate proton transfer, electron transfer, or nucleophilic/electrophilic attack, depending on the nature of the reaction.
- Dynamic Nature: Contrary to early models, the active site is not a rigid structure. It’s highly dynamic, undergoing conformational changes upon substrate binding. This flexibility is crucial for the "induced fit" model of enzyme-substrate interaction.
Beyond the Active Site: Allosteric Sites and Other Binding Regions
While the active site is the primary location for substrate binding, other regions on the enzyme can also play important roles in modulating enzyme activity. These include:
Allosteric Sites:
Allosteric sites are distinct binding sites located away from the active site. Binding of a molecule (an allosteric effector) to an allosteric site can induce conformational changes in the enzyme, affecting its ability to bind substrate and/or catalyze the reaction. Allosteric effectors can be either activators, enhancing enzyme activity, or inhibitors, reducing it. This allosteric regulation plays a vital role in controlling metabolic pathways and cellular responses to changing conditions. Allosteric regulation is crucial for maintaining homeostasis and responsiveness to environmental stimuli within a cell. This regulation ensures that enzyme activity aligns with the cell's immediate needs.
Binding Sites for Cofactors and Coenzymes:
Many enzymes require the assistance of cofactors or coenzymes to function effectively. These non-protein molecules can bind to specific sites on the enzyme, often within or near the active site, contributing to catalysis or stabilizing the enzyme structure. Cofactors can be metal ions (e.g., zinc, magnesium), while coenzymes are typically organic molecules derived from vitamins (e.g., NAD+, FAD). The binding of these accessory molecules is essential for the correct functioning of many enzymes. The specific location of cofactor binding within the enzyme's structure can influence both enzyme activity and substrate affinity.
Substrate Binding Exosites:
Some enzymes possess additional binding sites, called exosites, located outside the active site. These exosites can bind regulatory molecules or secondary substrates, affecting enzyme activity or specificity. Binding at these exosites can influence the conformation of the active site, either directly or indirectly impacting substrate binding and the catalytic process. This allows for a sophisticated level of regulation beyond simple allosteric control.
Models of Enzyme-Substrate Interaction
Two primary models explain how enzymes bind their substrates:
The Lock-and-Key Model:
This classical model proposed that the enzyme's active site has a rigid, pre-formed structure that precisely matches the shape of the substrate, like a lock and key. While conceptually simple, this model fails to account for the flexibility of enzymes and the conformational changes that often occur upon substrate binding.
The Induced-Fit Model:
The induced-fit model is a more accurate representation of enzyme-substrate interaction. In this model, the enzyme's active site is not a rigid structure but is flexible and undergoes conformational changes upon substrate binding. The substrate's binding induces a change in the enzyme's conformation, optimizing the active site for catalysis. This conformational change often involves the precise alignment of catalytic residues to facilitate the reaction. This dynamic interaction ensures that the enzyme adapts optimally to its specific substrate, enhancing the efficiency and specificity of catalysis.
Factors Affecting Substrate Binding
Several factors influence the strength and specificity of substrate binding:
- Shape and Charge Complementarity: The substrate must possess a shape and charge distribution that are complementary to the active site. This complementarity is essential for the formation of multiple weak interactions that hold the substrate in place.
- Hydrophobic and Hydrophilic Interactions: Hydrophobic residues within the active site can interact favorably with hydrophobic regions of the substrate, while hydrophilic residues can interact with polar or charged groups. The balance of these interactions plays a critical role in determining substrate binding affinity.
- Hydrogen Bonding: Hydrogen bonds are crucial for the precise orientation of the substrate within the active site, enhancing binding affinity and guiding the catalytic process.
- Ionic Interactions: Electrostatic interactions between charged residues in the active site and charged groups on the substrate can contribute significantly to substrate binding.
- Van der Waals Forces: These weak interactions, arising from temporary fluctuations in electron distribution, add cumulatively to the overall binding energy.
Studying Enzyme-Substrate Binding: Techniques and Approaches
Several experimental techniques allow researchers to study enzyme-substrate binding:
- X-ray Crystallography: This technique reveals the three-dimensional structure of the enzyme, including the active site and its interactions with the substrate. This provides a detailed, static view of the enzyme-substrate complex.
- NMR Spectroscopy: Nuclear magnetic resonance (NMR) spectroscopy provides dynamic information about the enzyme and its interaction with the substrate, revealing conformational changes upon binding.
- Surface Plasmon Resonance (SPR): This technique measures the binding affinity between an enzyme and its substrate in real time, providing quantitative data on the interaction strength.
- Fluorescence Spectroscopy: Fluorescence techniques can be used to monitor conformational changes in the enzyme upon substrate binding.
- Computational Methods: Molecular dynamics simulations and docking studies allow researchers to model enzyme-substrate interactions and predict binding affinities.
Conclusion
The precise location where a substrate binds to an enzyme is of paramount importance for enzyme function. The active site, a highly specialized region of the enzyme, is the primary binding location. However, other regions, such as allosteric sites and exosites, can also modulate binding and activity. The interplay of various interactions, the dynamic nature of the enzyme, and the use of advanced experimental techniques contribute to our continuous understanding of this fundamental biological process. This understanding is crucial not only for fundamental biological research but also for developing new drugs and therapeutic strategies targeting enzyme activity. Continued research into these intricate interactions will undoubtedly lead to further breakthroughs in our understanding of enzymes and their roles in life's processes.
Latest Posts
Latest Posts
-
The Ends Of Long Bones Are Called The
Mar 31, 2025
-
Is Ph A Chemical Or Physical Property
Mar 31, 2025
-
Identify The Characteristics Of The Hydroboration Oxidation Of An Alkene
Mar 31, 2025
-
What Are The Differences Between The Pulmonary And Systemic Circulation
Mar 31, 2025
-
A Perfectly Elastic Supply Curve Is
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Which Place On An Enzyme Binds A Substrate . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
