Why Are Covalent Bonds Soluble In Water
Muz Play
Mar 21, 2025 · 6 min read
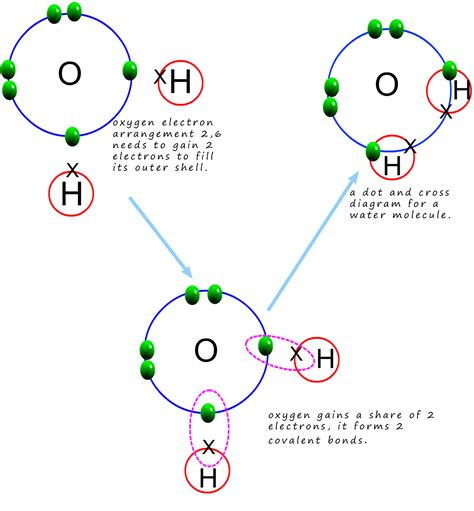
Table of Contents
Why Aren't Covalent Bonds Always Soluble in Water? Understanding Polarity and Intermolecular Forces
Covalent compounds, formed by the sharing of electrons between atoms, exhibit a wide range of solubility in water. While many believe that covalent compounds are inherently insoluble in water, this is a significant oversimplification. The truth is far more nuanced, depending heavily on the polarity of the molecule and the interplay of various intermolecular forces. This article delves deep into the factors governing the solubility of covalent compounds in water, dispelling common misconceptions and providing a comprehensive understanding of this crucial concept in chemistry.
The Role of Water's Polarity
Water (H₂O) is a highly polar molecule. This means that the electrons involved in the covalent bonds between oxygen and hydrogen are not shared equally. The oxygen atom, being more electronegative, attracts the shared electrons more strongly, creating a partial negative charge (δ-) on the oxygen and partial positive charges (δ+) on the hydrogens. This uneven distribution of charge creates a dipole moment, making water an excellent solvent for other polar molecules.
Polar Covalent Compounds and Water: A Match Made in Heaven (Sometimes)
Polar covalent compounds, like water itself, possess a dipole moment due to the unequal sharing of electrons between atoms of differing electronegativity. The positive end of one polar molecule is attracted to the negative end of another, leading to strong dipole-dipole interactions. When a polar covalent compound is introduced to water, these dipole-dipole interactions between the solute and solvent molecules are the primary driving force for solubility.
The stronger the dipole moment of the covalent compound, the greater its solubility in water. For instance, ethanol (C₂H₅OH), with its hydroxyl (-OH) group, is highly soluble in water due to the strong hydrogen bonding between the -OH group of ethanol and the water molecules. The oxygen atom in the -OH group carries a partial negative charge, while the hydrogen carries a partial positive charge, enabling strong attractive forces with water molecules.
Examples of Polar Covalent Compounds Soluble in Water:
- Sugars (e.g., glucose, sucrose): These molecules contain numerous hydroxyl (-OH) groups, leading to extensive hydrogen bonding with water.
- Amino acids: The presence of both acidic (-COOH) and basic (-NH₂) groups contributes to their polar nature and high solubility in water.
- Many inorganic acids and bases: Compounds like hydrochloric acid (HCl) and sodium hydroxide (NaOH), while ionic in solution, initially exist as polar covalent molecules.
Non-Polar Covalent Compounds and Water: A Tale of Two Worlds
Non-polar covalent compounds, on the other hand, have an even distribution of electrons within their molecules. They lack a significant dipole moment and consequently, have weak intermolecular forces, primarily London Dispersion Forces (LDFs). These weak forces are insufficient to overcome the strong hydrogen bonds between water molecules. As a result, non-polar covalent compounds tend to be insoluble in water. The water molecules are far more attracted to each other than they are to the non-polar solute, causing the non-polar substance to clump together, forming a separate phase.
Examples of Non-Polar Covalent Compounds Insoluble in Water:
- Oils and fats: These are long chains of hydrocarbons with relatively weak LDFs.
- Many hydrocarbons (e.g., methane, octane): These consist entirely of carbon and hydrogen atoms with similar electronegativities.
- Non-polar organic solvents (e.g., hexane, benzene): These lack significant polarity and predominantly exhibit LDFs.
The "Like Dissolves Like" Rule
The solubility of covalent compounds in water is best explained by the principle of "like dissolves like." Polar solvents, such as water, dissolve polar solutes, while non-polar solvents dissolve non-polar solutes. This principle highlights the importance of matching the intermolecular forces between the solute and solvent for successful dissolution.
The Influence of Molecular Size and Shape
Beyond polarity, the size and shape of a covalent molecule also play a role in its solubility. Larger molecules, even if polar, may have only a small proportion of their surface area involved in strong interactions with water. The rest of the molecule is dominated by weaker interactions, leading to decreased solubility. Similarly, the shape of the molecule can affect its ability to interact with water molecules. A molecule with a compact, symmetrical shape might be less soluble than a molecule with an elongated, asymmetric shape that can better interact with water's polar regions.
Beyond Polarity: Hydrogen Bonding – The Superstar Interaction
Hydrogen bonding is a special type of dipole-dipole interaction that occurs when a hydrogen atom is bonded to a highly electronegative atom (such as oxygen, nitrogen, or fluorine) and is attracted to another electronegative atom in a different molecule. This type of bonding is particularly strong and is crucial for the solubility of many covalent compounds in water. The more hydrogen bonding sites a molecule possesses, the greater its likelihood of being soluble in water.
Factors Affecting Solubility: Temperature and Pressure
Temperature and pressure also influence the solubility of covalent compounds in water. Generally, increasing the temperature increases the solubility of most solids and liquids in water. However, the effect on gases is often the opposite: increasing temperature decreases the solubility of gases in water. The effect of pressure is more significant for gases; increasing pressure increases the solubility of gases in water. These effects are due to changes in the kinetic energy of molecules and the balance between intermolecular forces.
Exceptions and Complexities
While the "like dissolves like" rule provides a useful guideline, there are always exceptions. Some molecules exhibit unexpected solubility behavior due to complex interplay of various factors like steric hindrance, conformational changes, and intramolecular hydrogen bonding. These exceptions highlight the need for a deeper understanding of the specific molecular interactions at play.
Applications and Importance
Understanding the solubility of covalent compounds in water is crucial in many fields:
- Medicine: The solubility of drugs affects their absorption and distribution in the body. Many drugs are designed to be soluble in water to facilitate their delivery to target sites.
- Environmental Science: The solubility of pollutants in water determines their transport and fate in the environment. Understanding solubility helps predict the potential impact of pollutants on aquatic ecosystems.
- Industrial Chemistry: Many industrial processes rely on the solubility of different compounds in water for separation, purification, and reaction control.
Conclusion
The solubility of covalent compounds in water is not a simple yes or no answer. It is a complex phenomenon determined by the interplay of several factors, primarily the polarity of the molecule, the strength of intermolecular forces, molecular size and shape, and external conditions like temperature and pressure. While the "like dissolves like" rule provides a useful starting point, a comprehensive understanding requires considering the specific interactions between the solute and solvent molecules. This understanding is fundamental across diverse scientific and technological disciplines, impacting areas from drug design to environmental protection and industrial processes. The intricate dance between polarity, hydrogen bonding, and other intermolecular forces continues to fascinate and challenge chemists, constantly pushing the boundaries of our understanding of solubility.
Latest Posts
Latest Posts
-
What Is The Cleft Of Venus
Mar 21, 2025
-
Describe Internal Factors Of Decision Making
Mar 21, 2025
-
How Does Thermal Energy Affect The 3 States Of Matter
Mar 21, 2025
-
Is Solubility A Chemical Or Physical Property
Mar 21, 2025
-
What Are Vertical Columns On The Periodic Table Called
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Why Are Covalent Bonds Soluble In Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
