Why Do Ionic Compounds Become Electrolytes
Muz Play
Mar 21, 2025 · 6 min read
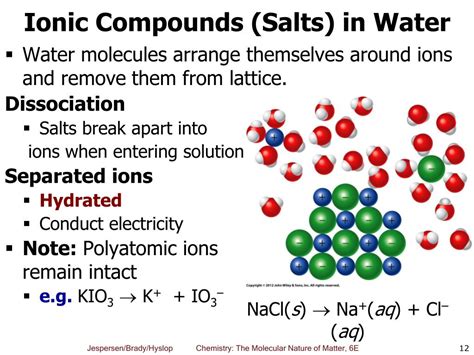
Table of Contents
Why Do Ionic Compounds Become Electrolytes?
Ionic compounds, characterized by the electrostatic attraction between oppositely charged ions, exhibit a unique property: their ability to conduct electricity when dissolved in water or melted. This conductivity stems from the presence of freely moving charged particles, known as ions, which are responsible for carrying an electric current. This article delves into the fundamental reasons behind this behavior, exploring the process of dissociation, the role of solvents, and the factors affecting the conductivity of ionic compounds.
Understanding Ionic Compounds and Their Structure
Before we explore their electrolytic behavior, let's establish a firm understanding of what ionic compounds are and how their structure contributes to their properties.
The Nature of Ionic Bonds
Ionic compounds are formed through the electrostatic attraction between positively charged ions (cations) and negatively charged ions (anions). This attraction arises from the transfer of electrons from a metal atom to a nonmetal atom, resulting in the formation of ions with complete electron shells. The strong electrostatic forces holding these ions together form the ionic bond, resulting in a highly ordered crystalline structure. These bonds are significantly stronger than the intermolecular forces found in covalent compounds, leading to high melting and boiling points. Examples of common ionic compounds include sodium chloride (NaCl), potassium iodide (KI), and magnesium oxide (MgO).
Crystalline Structure and Lattice Energy
The ions in ionic compounds are arranged in a highly ordered, three-dimensional lattice structure. This arrangement minimizes the repulsive forces between ions of the same charge while maximizing the attractive forces between ions of opposite charges. The strength of this lattice structure is quantified by lattice energy, which represents the energy required to completely separate one mole of a solid ionic compound into its constituent gaseous ions. A high lattice energy signifies a strong ionic bond and consequently, a high melting point. The strength of this lattice is crucial in understanding why ionic compounds need specific conditions to become electrolytes.
The Process of Dissociation and Ion Formation
The key to understanding why ionic compounds become electrolytes lies in the process of dissociation. This is the process where the ionic compound breaks apart into its constituent ions when dissolved in a suitable solvent or melted.
Dissociation in Aqueous Solutions
When an ionic compound is dissolved in water (a polar solvent), the water molecules interact with the ions in the crystal lattice. The partially positive hydrogen atoms of water molecules are attracted to the anions, while the partially negative oxygen atoms are attracted to the cations. This interaction weakens the electrostatic forces holding the ions together in the lattice. The water molecules effectively surround each ion, forming a hydration shell. This process, called solvation, stabilizes the ions in solution and prevents them from recombining. The hydrated ions are now free to move independently, enabling the conduction of electricity.
For example, when sodium chloride (NaCl) dissolves in water, it dissociates into sodium cations (Na⁺) and chloride anions (Cl⁻). The equation representing this dissociation is:
NaCl(s) → Na⁺(aq) + Cl⁻(aq)
The (aq) notation indicates that the ions are surrounded by water molecules (aqueous solution).
Dissociation in the Molten State
Ionic compounds can also conduct electricity when melted. In this state, the high temperature overcomes the electrostatic forces holding the ions together in the solid lattice. The ions become mobile, allowing for the flow of electric current. This explains why molten ionic compounds, such as molten sodium chloride, are excellent conductors of electricity.
The Role of the Solvent: Polarity and Dielectric Constant
The choice of solvent plays a critical role in the ability of an ionic compound to dissociate and become an electrolyte. Polar solvents, such as water, are essential for effective dissociation. The polarity of water, arising from the unequal sharing of electrons between oxygen and hydrogen atoms, enables it to interact effectively with the ions. The high dielectric constant of water also significantly reduces the electrostatic attraction between the ions, facilitating dissociation.
Non-Polar Solvents and Ion Pairing
In contrast, non-polar solvents, such as hexane or benzene, have a low dielectric constant and lack the polar nature to effectively solvate ions. As a result, ionic compounds generally do not dissolve well in these solvents, and even if they do dissolve slightly, the ions tend to remain associated as ion pairs, hindering their ability to conduct electricity. The limited movement of ions restricts electrical conductivity.
Factors Affecting Conductivity
Several factors influence the extent to which an ionic compound will conduct electricity when dissolved or melted.
Concentration of Ions
The conductivity of an electrolytic solution is directly proportional to the concentration of ions present. A higher concentration of ions means more charge carriers are available to conduct electricity, leading to increased conductivity.
Temperature
Temperature plays a crucial role in the conductivity of ionic solutions. Increasing the temperature increases the kinetic energy of the ions, enhancing their mobility and leading to improved conductivity. This increased mobility overcomes the inter-ionic attractions to a larger extent.
Nature of the Ionic Compound
The nature of the ionic compound itself affects its conductivity. Compounds with highly charged ions (e.g., Mg²⁺, Al³⁺) tend to exhibit stronger electrostatic attractions, leading to lower dissociation and reduced conductivity compared to compounds with singly charged ions (e.g., Na⁺, K⁺). Similarly, the size of the ions also plays a role; smaller ions generally have stronger electrostatic interactions, resulting in lower conductivity.
Electrolytes and Non-Electrolytes: A Comparison
Electrolytes are substances that conduct electricity when dissolved in water or melted, while non-electrolytes do not. The fundamental difference lies in the ability of the substance to dissociate into ions.
Ionic compounds are typically strong electrolytes, meaning they dissociate completely or almost completely into ions in solution. This complete dissociation leads to high conductivity. Weak electrolytes, on the other hand, only partially dissociate into ions, resulting in lower conductivity. Examples include weak acids and bases like acetic acid (CH₃COOH) and ammonia (NH₃). Non-electrolytes, such as sugar (sucrose) and ethanol, do not dissociate into ions and thus do not conduct electricity.
Applications of Electrolytes
The electrolytic properties of ionic compounds have numerous practical applications in various fields:
-
Batteries: Electrolytes are crucial components of batteries, facilitating the movement of ions between the electrodes, generating an electric current.
-
Electroplating: Electrolytes are used in electroplating processes to deposit a thin layer of metal onto another surface.
-
Electrolysis: Electrolytes are essential in electrolysis processes, where an electric current is passed through a solution to drive chemical reactions.
-
Medical Applications: Electrolyte solutions are vital in medical applications, such as intravenous fluids, to maintain the proper balance of ions in the body.
-
Industrial Processes: Electrolytes are used in various industrial processes, including metal refining and water treatment.
Conclusion
The ability of ionic compounds to become electrolytes is a direct consequence of their ionic structure and their capacity to dissociate into freely moving ions when dissolved in a suitable solvent or melted. The process of dissociation is facilitated by the interaction between the ions and the polar solvent molecules, which weakens the electrostatic forces holding the ions together in the crystal lattice. Several factors, including concentration, temperature, and the nature of the ionic compound, influence the extent of conductivity. Understanding the electrolytic behavior of ionic compounds is crucial in various applications across diverse scientific and technological fields.
Latest Posts
Latest Posts
-
Standard Error For Difference In Means
Mar 22, 2025
-
Definition Of Reference Group In Sociology
Mar 22, 2025
-
The Study Of The Geographical Distribution Of Organisms Is Called
Mar 22, 2025
-
Lewis Dot Structures For Polyatomic Ions
Mar 22, 2025
-
Is Tap Water A Homogeneous Mixture
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about Why Do Ionic Compounds Become Electrolytes . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
