Why Is Rusting A Chemical Change
Muz Play
Mar 30, 2025 · 5 min read
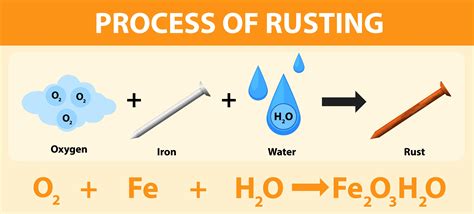
Table of Contents
Why is Rusting a Chemical Change? A Deep Dive into Oxidation and Redox Reactions
Rusting, that familiar orange-brown coating on iron and steel, is more than just a cosmetic blemish. It's a compelling example of a chemical change, a process that fundamentally alters the composition of a substance, forming new materials with different properties. Understanding why rusting falls squarely into this category requires exploring the fascinating world of oxidation, reduction, and redox reactions.
Understanding Chemical Changes vs. Physical Changes
Before delving into the specifics of rusting, let's establish a clear distinction between chemical and physical changes. A physical change alters the form or appearance of a substance but doesn't change its chemical composition. Think of melting ice: it changes from a solid to a liquid, but it remains water (H₂O). The molecules themselves remain intact.
A chemical change, on the other hand, involves the rearrangement of atoms and molecules, forming entirely new substances with different properties. This often involves breaking and forming chemical bonds. Burning wood is a classic example: the wood is transformed into ash, smoke, and gases, all distinctly different substances from the original wood.
Rusting: A Redox Reaction in Action
Rusting, or the oxidation of iron, is unequivocally a chemical change. It's a complex process involving a redox reaction, short for reduction-oxidation reaction. Redox reactions involve the transfer of electrons between atoms or ions. One substance loses electrons (oxidation), while another substance gains electrons (reduction). These two processes always occur simultaneously.
The Role of Oxygen and Water
The rusting of iron (Fe) requires both oxygen (O₂) and water (H₂O). Oxygen acts as the oxidizing agent, accepting electrons from iron, while water provides the medium for the reaction to occur and participates directly in the chemical process.
The overall reaction can be simplified as:
4Fe(s) + 3O₂(g) + 6H₂O(l) → 4Fe(OH)₃(s)
This simplified equation shows iron (Fe) reacting with oxygen (O₂) and water (H₂O) to form iron(III) hydroxide, Fe(OH)₃, which is a component of rust. However, the actual process is significantly more complex, involving several intermediate steps and the formation of various iron oxides and hydroxides.
The Oxidation of Iron
In the rusting process, iron atoms lose electrons, a process known as oxidation. Iron, in its metallic state, has a zero oxidation state. Upon losing electrons, it transitions to a higher oxidation state, typically +2 (ferrous) or +3 (ferric). The electrons lost by the iron are gained by the oxygen atoms.
Fe → Fe²⁺ + 2e⁻ (Oxidation of iron to ferrous ion) Fe²⁺ → Fe³⁺ + e⁻ (Further oxidation of ferrous to ferric ion)
The Reduction of Oxygen
Simultaneously, oxygen molecules gain electrons, a process called reduction. The oxygen atoms in O₂ have an oxidation state of 0. After gaining electrons, they become part of hydroxide ions (OH⁻) or oxide ions (O²⁻).
O₂ + 4e⁻ + 2H₂O → 4OH⁻ (Reduction of oxygen to hydroxide ions)
Evidence of a Chemical Change in Rusting
Several key observations provide irrefutable evidence that rusting is a chemical change:
- Change in color: The most obvious sign is the change in color from the silvery-grey of iron to the reddish-brown of rust. This color change indicates the formation of new chemical compounds.
- Change in properties: Rust is brittle and flaky, unlike the relatively strong and malleable nature of iron. This difference in physical properties further underscores the transformation into a new substance.
- Irreversibility: Rust cannot be easily converted back into iron. While some processes can recover iron from its oxides, it's not a simple reversal of the rusting process. This irreversibility is a hallmark of a chemical change.
- Formation of new substances: The formation of iron oxides and hydroxides, which are chemically distinct from iron, is conclusive evidence of a chemical reaction. The new compounds have different chemical properties and compositions.
- Energy changes: Rusting is an exothermic process, releasing heat. While the heat release may be subtle, it confirms the involvement of chemical bond breaking and formation, characteristic of chemical reactions.
Factors Affecting the Rate of Rusting
The rate at which iron rusts is influenced by several environmental factors:
- Exposure to oxygen: A higher concentration of oxygen accelerates rusting, as oxygen is a crucial reactant in the redox reaction.
- Exposure to water: Water acts as an electrolyte, facilitating the movement of ions and accelerating the electron transfer process. Higher humidity and the presence of salt water significantly increase the rate of rusting.
- Temperature: Higher temperatures generally increase the rate of chemical reactions, including rusting.
- Presence of electrolytes: Substances that dissolve in water to form ions, such as salts, increase the conductivity of water and speed up the rusting process. This is why saltwater environments are particularly corrosive to iron.
- Surface area: A larger surface area of iron exposed to the environment results in faster rusting as more iron atoms are available to react with oxygen and water.
Preventing Rust: A Battle Against Oxidation
Given the detrimental effects of rusting, considerable effort is dedicated to preventing it. Strategies for rust prevention include:
- Protective coatings: Applying paint, varnish, or other coatings creates a barrier between iron and the environment, preventing contact with oxygen and water.
- Galvanization: Coating iron with zinc protects it from rusting through sacrificial corrosion. Zinc oxidizes preferentially, protecting the underlying iron.
- Alloying: Adding other metals to iron, such as chromium (stainless steel), alters its chemical properties and reduces its susceptibility to rusting.
- Cathodic protection: This technique uses a more active metal (like zinc or magnesium) to act as a sacrificial anode, protecting the iron from oxidation.
- Controlling the environment: Reducing exposure to moisture and oxygen, for example, in dry, airtight conditions, minimizes rust formation.
Conclusion: Rusting as a Definitive Chemical Change
In conclusion, the evidence overwhelmingly supports the classification of rusting as a chemical change. The transformation of iron into rust involves a redox reaction, resulting in the formation of new substances with different chemical and physical properties. This process is irreversible, involves energy changes, and is significantly influenced by environmental factors. Understanding the chemical nature of rusting is crucial for developing effective strategies for its prevention and mitigation in various applications. From protecting bridges and pipelines to preserving antique ironwork, combating rust remains a critical endeavor that relies on a deep understanding of its fundamental chemical processes. The complex interplay of oxygen, water, and iron, leading to the formation of rust, serves as a compelling illustration of the dynamic and transformative power of chemical reactions in our everyday world.
Latest Posts
Latest Posts
-
What Is The Definition Of Form In Music
Apr 01, 2025
-
Staph Epidermidis Hemolysis On Blood Agar
Apr 01, 2025
-
Difference Between Autonomic And Somatic Nervous System
Apr 01, 2025
-
Phase Change Properties Of Pure Substances
Apr 01, 2025
-
Where Do Antimicrobial Drugs Come From
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Why Is Rusting A Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
