Why Must The Glucoisomerase Be Opened Before Phosphorylation
Muz Play
Mar 18, 2025 · 5 min read
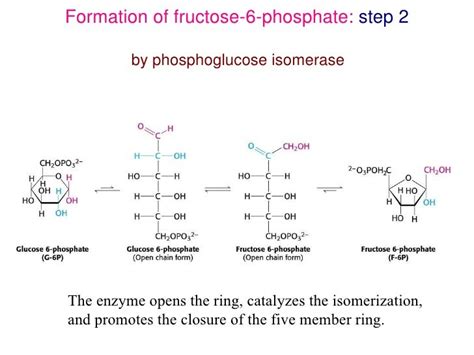
Table of Contents
Why Must Glucoisomerase Be Opened Before Phosphorylation? A Deep Dive into Enzyme Structure and Function
Understanding the intricacies of enzyme catalysis is crucial in various fields, from medicine and biotechnology to industrial processes. One fascinating example lies in the behavior of glucoisomerase, an enzyme pivotal in the conversion of glucose to fructose, a process extensively used in the food industry to produce high-fructose corn syrup. This article delves into the critical necessity of glucoisomerase's open conformation before phosphorylation can occur, exploring the underlying structural mechanisms and their functional implications.
The Role of Glucoisomerase in Glucose-Fructose Interconversion
Glucoisomerase, also known as xylose isomerase, is a crucial enzyme catalyzing the reversible isomerization between glucose and fructose. This reaction is of significant industrial importance, primarily in the production of high-fructose corn syrup (HFCS), a prevalent sweetener in many processed foods and beverages. The enzyme's ability to efficiently convert glucose, a less sweet sugar, into fructose, a much sweeter one, makes it a cornerstone of the food industry. Understanding its mechanism, especially the conformational changes required for its activity, is essential for optimizing industrial processes and developing improved enzyme variants.
The Mechanism of Glucoisomerase Action: A Step-by-Step Look
The catalytic mechanism of glucoisomerase involves several key steps:
-
Substrate Binding: The glucose molecule binds to the active site of the enzyme. This binding initiates a conformational change within the enzyme.
-
Isomerization: Once bound, the glucose molecule undergoes isomerization through a series of complex steps involving proton abstraction, hydride shift, and proton donation. This process results in the conversion of glucose to fructose.
-
Product Release: The newly formed fructose molecule is released from the active site, allowing the enzyme to bind another glucose molecule and repeat the catalytic cycle.
The Significance of Conformational Changes: From Closed to Open
Glucoisomerase, like many enzymes, exhibits a dynamic conformational landscape. This means it exists in different three-dimensional structures, each playing a specific role in the catalytic cycle. Two major conformations are particularly relevant: the closed conformation and the open conformation.
-
Closed Conformation: In this state, the active site is enclosed, creating a tightly bound environment optimized for substrate binding and catalysis. The enzyme essentially “clamps down” on the substrate, facilitating the isomerization process.
-
Open Conformation: In contrast, the open conformation presents a more accessible active site. This state is crucial for substrate entry and product release. The enzyme adopts a more relaxed structure, allowing for the efficient association and dissociation of molecules.
The Importance of the Open Conformation Before Phosphorylation
Phosphorylation, the covalent attachment of a phosphate group to a protein, is a widespread post-translational modification that often regulates enzyme activity. In the case of glucoisomerase, phosphorylation's role is complex and deeply intertwined with its conformational dynamics. The open conformation is essential before phosphorylation can occur effectively for several reasons:
-
Accessibility of Phosphorylation Sites: Phosphorylation sites, typically serine, threonine, or tyrosine residues, must be accessible to the kinase enzyme responsible for catalyzing the phosphorylation reaction. In the closed conformation, these sites might be buried within the protein's core, hindering the access of the kinase. The open conformation exposes these sites, making them readily available for phosphorylation.
-
Optimal Interaction with Kinases: The open conformation might induce conformational changes that create a suitable binding site for the kinase. This interaction is vital for effective phosphorylation. The closed conformation might sterically hinder the interaction between the glucoisomerase and the kinase.
-
Regulation of Enzyme Activity: Phosphorylation can either activate or inhibit enzyme activity, depending on the specific enzyme and the location of the phosphorylation site. By regulating the transition between open and closed conformations, phosphorylation acts as a switch, controlling glucoisomerase's catalytic ability. The open conformation may present a suitable environment for a kinase to bind and phosphorylate a specific residue that then promotes a transition to a catalytically active state (or vice versa).
Structural Insights: Understanding the Molecular Mechanisms
The specific structural details underlying the relationship between glucoisomerase's conformation and phosphorylation are complex and require advanced techniques like X-ray crystallography and NMR spectroscopy to elucidate. However, general principles can be outlined:
-
Flexibility of Loops and Domains: Regions of the glucoisomerase protein, such as loops and domains, exhibit considerable flexibility. These regions undergo significant conformational changes during the transition between open and closed states, exposing or hiding phosphorylation sites.
-
Allosteric Regulation: Phosphorylation might induce allosteric effects, meaning that the modification at one site influences the conformation and activity at another, distant site. This long-range effect can regulate the enzyme's overall structure and function.
-
Interaction with Chaperones: Molecular chaperones might play a role in guiding the enzyme's transition between different conformational states. Chaperones could assist in maintaining the correct conformation for optimal phosphorylation or subsequently for catalytic activity.
Implications and Future Directions
The intricate interplay between glucoisomerase's conformational dynamics and phosphorylation highlights the complexity of enzyme regulation. This knowledge has significant implications in:
-
Metabolic Engineering: Understanding the factors influencing glucoisomerase activity can be exploited to engineer strains with improved catalytic properties, leading to higher yields in industrial processes.
-
Drug Discovery: Inhibitors or activators targeting specific conformations of glucoisomerase could offer novel therapeutic avenues for metabolic diseases.
-
Enzyme Design: By understanding the structural basis for conformational changes, researchers can design new enzyme variants with enhanced stability, activity, and regulation.
Further research is needed to fully unravel the precise molecular mechanisms governing the relationship between glucoisomerase conformation and phosphorylation. Advanced structural biology techniques coupled with computational modeling offer promising avenues to gain deeper insights. A comprehensive understanding of these processes has far-reaching implications for various biotechnological applications and our understanding of fundamental biological processes. The ability to precisely control enzyme activity through targeted manipulation of its conformational states represents a significant step forward in the development of next-generation biotechnologies.
Conclusion: The Open Conformation – A Gateway to Regulation
The requirement for glucoisomerase to adopt an open conformation before phosphorylation underscores the dynamic nature of enzyme regulation. This conformational change exposes critical phosphorylation sites, allowing for proper kinase interaction and subsequent modulation of enzyme activity. Understanding the structural basis of this relationship is crucial for optimizing enzyme function in industrial applications, developing novel therapeutic strategies, and advancing our understanding of fundamental biochemical mechanisms. As research continues to unveil the intricate details of this process, it holds immense promise for a wide range of applications. The open conformation, therefore, is not merely a structural state but a critical gateway to the sophisticated regulatory mechanisms governing glucoisomerase's crucial role in glucose metabolism and industrial processes.
Latest Posts
Latest Posts
-
A Relationship Between Two Organisms In Which Both Organisms Benefit
Mar 18, 2025
-
How To Compute Cost Per Equivalent Unit
Mar 18, 2025
-
What Are The Physical Properties Of A Metal
Mar 18, 2025
-
Why Is The Force Subscript Not Written In The Us
Mar 18, 2025
-
A Relationship In Which Both Organisms Benefit
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Why Must The Glucoisomerase Be Opened Before Phosphorylation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
