Write The Condensed Structure For Each Of These Skeletal Structures
Muz Play
Mar 29, 2025 · 5 min read
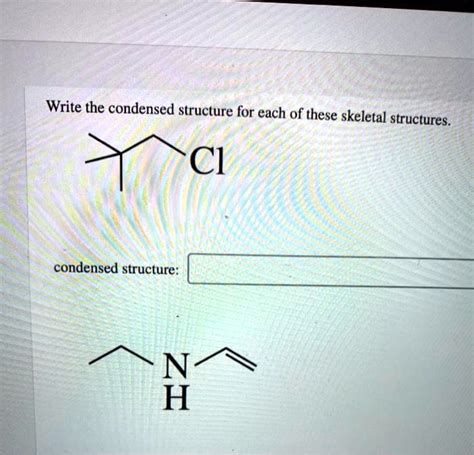
Table of Contents
Deciphering Skeletal Structures: A Comprehensive Guide to Condensed Structural Formulas
Understanding the structure of organic molecules is fundamental to chemistry. While complete skeletal structures provide a detailed visual representation, condensed structural formulas offer a more concise and efficient way to depict the same information. This article will guide you through the process of converting complex skeletal structures into their condensed counterparts, covering various functional groups and complexities. We'll break down the process step-by-step, ensuring a clear understanding for both beginners and those seeking to refine their organic chemistry skills.
What are Skeletal Structures and Condensed Structural Formulas?
Before delving into the conversion process, let's define our terms.
Skeletal Structures: These diagrams use lines to represent carbon-carbon bonds, with carbon atoms implied at the ends and intersections of the lines. Hydrogen atoms attached to carbon are often omitted for simplicity, while other atoms (like oxygen, nitrogen, chlorine, etc.) are explicitly shown. Skeletal structures prioritize the visualization of the carbon backbone and the arrangement of other atoms.
Condensed Structural Formulas: These formulas show all atoms present in a molecule, but they often omit explicit depiction of bonds. Atoms connected to a central carbon are grouped together, simplifying the representation without losing crucial structural information. They are a more compact and written-out version of the skeletal structure.
Step-by-Step Conversion of Skeletal Structures to Condensed Formulas
Let's illustrate the conversion process with examples, focusing on different functional groups and complexities.
1. Simple Alkanes:
Consider a simple alkane like butane. Its skeletal structure is a straight chain of four carbons:
C-C-C-C
The condensed formula would be: CH₃CH₂CH₂CH₃ (or CH₃(CH₂)₂CH₃ for further simplification). Each carbon atom has its associated hydrogens explicitly shown.
2. Branched Alkanes:
Let's examine a branched alkane, 2-methylpropane:
CH₃
|
CH₃-C-CH₃
The condensed formula is: (CH₃)₃CH. Notice how the three methyl groups (CH₃) are grouped together, attached to the central carbon.
3. Alkenes and Alkynes:
Unsaturated hydrocarbons, containing double or triple bonds, require careful attention to the positioning of these bonds in the condensed formula. For example, propene:
CH₂=CH-CH₃
The condensed formula retains the double bond: CH₂=CHCH₃. Similarly, for propyne (containing a triple bond):
CH≡C-CH₃
The condensed formula is: CH≡CCH₃.
4. Alcohols and Ethers:
Oxygen-containing functional groups are also easily incorporated. For ethanol:
CH₃-CH₂-OH
The condensed formula is: CH₃CH₂OH. Similarly, for diethyl ether:
CH₃-CH₂-O-CH₂-CH₃
The condensed formula is: CH₃CH₂OCH₂CH₃.
5. Halogenated Alkanes:
Halogens (F, Cl, Br, I) are directly included in the condensed formula. For example, chloromethane:
CH₃-Cl
The condensed formula is: CH₃Cl. For 1,2-dichloroethane:
Cl-CH₂-CH₂-Cl
The condensed formula is: ClCH₂CH₂Cl.
6. Carboxylic Acids, Ketones, and Aldehydes:
These carbonyl-containing compounds are handled similarly, showing the carbonyl group (C=O). For example, acetic acid (ethanoic acid):
CH₃-COOH
The condensed formula is: CH₃COOH. For propanone (acetone):
CH₃-CO-CH₃
The condensed formula is: CH₃COCH₃. For propanal:
CH₃CH₂CHO
The condensed formula is: CH₃CH₂CHO
7. Amines:
Nitrogen-containing compounds are handled similarly, explicitly showing the nitrogen atom and its attachments. For example, methylamine:
CH₃-NH₂
The condensed formula is: CH₃NH₂. For dimethylamine:
CH₃-NH-CH₃
The condensed formula is: (CH₃)₂NH
8. More Complex Structures:
As molecular complexity increases, the condensed formula becomes a useful tool. For example, consider a molecule with a benzene ring:
(Image of a substituted benzene ring would be placed here, showing a methyl group and a hydroxyl group attached)
The condensed formula could be: C₆H₄(CH₃)(OH) (the exact position of the substituents needs to be specified, e.g., o-, m-, p- isomers).
Tips and Tricks for Efficient Conversion
- Start from the longest carbon chain: Identify the longest continuous carbon chain and write its condensed formula first.
- Add branches systematically: Add branches and functional groups step-by-step to avoid missing atoms or bonds.
- Use parentheses to clarify branching: Parentheses are crucial for denoting multiple identical substituents attached to a single carbon atom.
- Maintain the order of atoms: The order of atoms in the condensed formula reflects the bonding in the molecule.
- Practice: The best way to master the skill is through consistent practice. Start with simple structures and progressively increase the complexity.
Importance of Condensed Structural Formulas in Organic Chemistry
Condensed structural formulas play a vital role in organic chemistry for several reasons:
- Conciseness: They provide a compact representation of molecular structure, saving space and time, especially when dealing with large and complex molecules.
- Clarity: Despite their brevity, they clearly convey all the essential structural information.
- Efficiency: They are easier and faster to write than complete structural formulas, improving efficiency in note-taking and communication.
- Predicting properties: The condensed formula helps to predict the chemical and physical properties of the molecule based on its functional groups and structure.
- Nomenclature: Condensed formulas are often used as a starting point for naming organic compounds using IUPAC nomenclature.
Conclusion
Converting skeletal structures to condensed structural formulas is an essential skill in organic chemistry. By following the step-by-step process and utilizing the tips outlined in this article, you can confidently translate visual representations of molecules into their concise and informative condensed forms. This skill will greatly enhance your understanding and manipulation of organic molecules, paving the way for more advanced concepts and applications within the field. Consistent practice is key to mastering this crucial skill and becoming proficient in organic chemistry.
Latest Posts
Latest Posts
-
Lab Skills Using A Graduated Cylinder
Apr 01, 2025
-
Which Molecules In Eukaryotic Cells Regulate Gene Expression
Apr 01, 2025
-
How Do Natural Killer Cells Destroy Invading Pathogens
Apr 01, 2025
-
What Stimulates The Pollen Tube To Grow
Apr 01, 2025
-
How To Find C In Sinusoidal Function
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Write The Condensed Structure For Each Of These Skeletal Structures . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
