Your Job Is To Synthesize Non-4-yne
Muz Play
Mar 24, 2025 · 5 min read
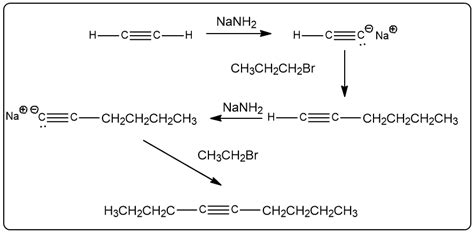
Table of Contents
Your Job is to Synthesize Non-4-yne: A Comprehensive Guide
Synthesizing non-4-ynes, specifically those lacking a triple bond at the 4-position, presents a unique synthetic challenge. This detailed guide explores various strategies, reaction mechanisms, and considerations for achieving this goal, emphasizing practicality and efficiency. We'll delve into different approaches, highlighting their advantages and disadvantages, enabling you to select the optimal method for your specific target molecule.
Understanding the Challenges of Non-4-yne Synthesis
The synthesis of non-4-ynes is often more complex than that of their 4-yne counterparts due to regioselectivity issues. Many standard alkyne synthesis methods can lead to the formation of the 4-yne as a major product, necessitating careful choice of reagents and reaction conditions. The position of the triple bond is crucial for the properties and biological activity of many molecules, making the selective synthesis of non-4-ynes a vital area of research.
Regioselectivity: The Key Hurdle
Achieving regioselectivity – controlling the position of the triple bond – is paramount. Traditional methods like elimination reactions or coupling reactions often lack the precision needed to consistently produce the desired non-4-yne isomer. This lack of regiocontrol often results in a mixture of isomers, requiring tedious and inefficient purification steps.
Strategies for Non-4-yne Synthesis
Several strategies can be employed to overcome the regioselectivity problem and efficiently synthesize non-4-ynes. These methods often involve building the carbon skeleton strategically, incorporating the alkyne functionality at a later stage, or utilizing protecting group strategies to control reactivity.
1. Protecting Group Strategies
Protecting groups are essential tools in organic synthesis, enabling the selective manipulation of functional groups. In the context of non-4-yne synthesis, protecting groups can prevent unwanted reactions at the 4-position or other sensitive sites, allowing selective alkyne formation elsewhere.
-
Examples: Silyl protecting groups (like TBDMS or TIPS) can protect alcohols or alkynes, preventing them from participating in unwanted reactions. These groups can be selectively removed at a later stage using appropriate reagents like fluoride ions.
-
Advantages: Provides excellent control over reactivity, minimizing the formation of undesired isomers.
-
Disadvantages: Adds extra steps to the synthesis, increasing the overall reaction time and potentially reducing the overall yield.
2. Strategic Carbon-Carbon Bond Formation
Building the carbon skeleton with the alkyne functionality already in place at the desired position is a powerful strategy. This avoids the regioselectivity issues associated with introducing the alkyne later in the synthesis.
-
Examples: Using appropriately substituted alkynes in coupling reactions (like Sonogashira or Cadiot-Chodkiewicz) to build the desired carbon framework. Utilizing ring-closing metathesis (RCM) reactions to create cyclic systems with the alkyne positioned correctly.
-
Advantages: Reduces the chance of forming undesired isomers, leading to improved yields and purity.
-
Disadvantages: Requires careful planning of the synthetic route and readily available starting materials with the alkyne in the correct position.
3. Directed Alkylation/Acylation
This approach uses directing groups to control the position of subsequent functionalization reactions. The directing group influences the regioselectivity of the reaction, leading to the preferential formation of the non-4-yne.
-
Examples: Utilizing a directing group like an ortho-substituent on an aromatic ring to guide alkyne formation to a specific position.
-
Advantages: Provides a high degree of regiocontrol, enhancing the selectivity of the reaction.
-
Disadvantages: Requires the selection of an appropriate directing group and its subsequent removal, adding complexity to the overall synthesis.
4. Rearrangement Reactions
Certain rearrangement reactions can be employed to isomerize an initially formed 4-yne to the desired non-4-yne isomer. These reactions typically involve catalysts or specific reaction conditions that promote the shift of the triple bond.
-
Examples: Base-catalyzed isomerization reactions, metal-catalyzed rearrangements.
-
Advantages: Can be a concise way to obtain the non-4-yne from a readily accessible 4-yne precursor.
-
Disadvantages: Often requires harsh reaction conditions and may lead to side reactions or low yields.
Choosing the Right Method: Factors to Consider
The optimal method for synthesizing a specific non-4-yne depends on several crucial factors:
-
Target molecule structure: The complexity and specific functional groups present in the target molecule will influence the choice of reaction strategy. Steric hindrance, the presence of sensitive functional groups, and the desired overall yield will all need to be considered.
-
Availability of starting materials: The choice of method will be influenced by the availability and cost of the necessary starting materials. Using readily available and cost-effective starting materials is vital for the economic feasibility of the synthesis.
-
Reaction conditions: The feasibility of the reaction will depend on the compatibility of the chosen reaction conditions with the substrate and other functional groups. This involves careful consideration of temperature, solvent, and reagent compatibility.
-
Yield and purity: The overall yield and purity of the final product are critical considerations. A high-yielding and selective method is always preferred to minimize waste and maximize efficiency.
Advanced Techniques and Future Directions
The field of non-4-yne synthesis is constantly evolving. Advanced techniques and future research directions are paving the way for more efficient and versatile methods:
-
Flow chemistry: Performing reactions in microfluidic devices offers superior control over reaction parameters and can improve yield and selectivity.
-
Catalyst development: Developing new catalysts with enhanced regioselectivity and activity is a crucial area of research. This involves exploring novel metal complexes and organocatalysts.
-
Computational chemistry: Computational methods can predict reaction pathways and optimize reaction conditions, significantly reducing the time and resources needed for experimental optimization.
-
Biocatalysis: Utilizing enzymes as catalysts offers a greener and more sustainable approach to non-4-yne synthesis, often with high regio- and stereoselectivity.
Conclusion: Mastering the Art of Non-4-yne Synthesis
The synthesis of non-4-ynes presents a significant challenge in organic chemistry. However, by carefully considering the available strategies, choosing appropriate reaction conditions, and employing advanced techniques, researchers can efficiently synthesize these valuable compounds. The selection of the optimal method demands a thorough understanding of the target molecule, available resources, and the limitations of each approach. As research continues, the development of even more efficient and sustainable methods for non-4-yne synthesis will undoubtedly enhance their applications in various fields, from pharmaceuticals to materials science. Mastering the art of non-4-yne synthesis requires careful planning, precise execution, and a deep understanding of organic chemistry principles.
Latest Posts
Latest Posts
-
Claim Of Fact Value And Policy
Mar 27, 2025
-
How To Calculate Shortage And Surplus
Mar 27, 2025
-
Is A Cricket A Vertebrate Or Invertebrate
Mar 27, 2025
-
What Do Colligative Properties Depend On
Mar 27, 2025
-
How To Write A Similarity Statement
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about Your Job Is To Synthesize Non-4-yne . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
