What Do Colligative Properties Depend On
Muz Play
Mar 27, 2025 · 6 min read
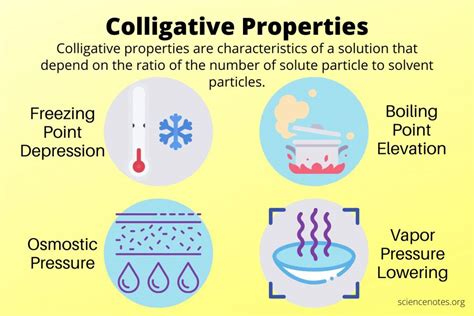
Table of Contents
What Do Colligative Properties Depend On? A Deep Dive into Solution Behavior
Colligative properties are a fascinating aspect of chemistry, describing how the physical properties of a solution change based on the concentration of solute particles, regardless of their identity. Understanding what these properties depend on is crucial for various applications, from designing efficient antifreeze solutions to predicting osmotic pressure in biological systems. This comprehensive guide delves into the intricacies of colligative properties, exploring the factors that influence them and their significance in different fields.
The Essence of Colligative Properties
Colligative properties are characteristics of a solution that depend solely on the number of solute particles present, not their identity or chemical nature. This means that whether you dissolve 1 mole of glucose or 1 mole of sucrose in a given amount of water, the effect on the colligative properties will be essentially the same (assuming complete dissociation). The key is the number of particles, not their type. This is fundamentally different from other solution properties, like color or viscosity, which are directly influenced by the chemical nature of the solute.
There are four primary colligative properties:
- Vapor Pressure Lowering: The presence of a non-volatile solute reduces the vapor pressure of the solvent.
- Boiling Point Elevation: The boiling point of a solution is higher than that of the pure solvent.
- Freezing Point Depression: The freezing point of a solution is lower than that of the pure solvent.
- Osmotic Pressure: The pressure required to prevent the flow of solvent across a semi-permeable membrane separating the solution and the pure solvent.
Factors Influencing Colligative Properties: The Number Game
As emphasized earlier, the core factor determining the magnitude of colligative properties is the number of solute particles present in the solution. This leads us to several key aspects:
1. Concentration of Solute: The More, the Merrier (or the More Pronounced Effect)
The concentration of solute is directly proportional to the magnitude of colligative properties. A higher concentration of solute particles means a greater impact on the vapor pressure, boiling point, freezing point, and osmotic pressure. This is typically expressed in terms of molality (moles of solute per kilogram of solvent), as molality is independent of temperature, unlike molarity.
Example: A 1 molal solution of NaCl will exhibit a greater effect on freezing point depression than a 0.5 molal solution of NaCl because it has a higher concentration of solute particles.
2. Dissociation of Ionic Compounds: The Power of Ions
The number of particles dramatically increases when dealing with ionic compounds that dissociate in solution. For instance, NaCl dissociates into Na⁺ and Cl⁻ ions. A 1 molal solution of NaCl will effectively have twice the number of particles (approximately, considering activity coefficients) compared to a 1 molal solution of a non-electrolyte like glucose, which does not dissociate. This leads to a larger change in colligative properties.
The Van't Hoff Factor (i): To account for dissociation, the Van't Hoff factor (i) is introduced. It represents the number of particles produced per formula unit of solute when it dissolves. For NaCl, i is approximately 2; for MgCl₂, it's approximately 3, and so on. The actual value of i can deviate from the ideal value due to ion pairing and other interionic forces. The equations for colligative properties are then modified to include the Van't Hoff factor.
3. Association of Molecules: The Clustering Effect
Conversely, some molecules may associate in solution, forming larger clusters. This reduces the effective number of solute particles. For example, certain carboxylic acids can dimerize (form pairs) in nonpolar solvents, leading to a lower-than-expected change in colligative properties. The Van't Hoff factor in these cases would be less than 1.
4. Nature of the Solvent: The Medium Matters
While colligative properties primarily depend on the solute, the nature of the solvent does play a role, primarily through its physical properties. The solvent's properties influence the strength of interactions between solute and solvent molecules, influencing the extent of dissociation or association and affecting the effective number of particles. The solvent's molar mass affects the molality calculation, altering the colligative property magnitude. Additionally, the dielectric constant of the solvent impacts ion-ion interactions in solutions containing ionic compounds.
5. Temperature: A Subtle Influence
Temperature affects the kinetic energy of the particles in the solution and thus indirectly influences colligative properties. Higher temperatures can increase the dissociation of some ionic compounds or lead to increased association of others, thus changing the effective number of particles. However, the primary effect of temperature is reflected in the solvent properties like vapor pressure, which then impact colligative properties.
Equations and Applications: Putting it all Together
The relationships between colligative properties and solute concentration are mathematically expressed through various equations:
-
Vapor Pressure Lowering: ΔP = X<sub>solute</sub> * P°<sub>solvent</sub> (Raoult's Law, assuming ideal solutions) where X<sub>solute</sub> is the mole fraction of the solute and P°<sub>solvent</sub> is the vapor pressure of the pure solvent.
-
Boiling Point Elevation: ΔT<sub>b</sub> = K<sub>b</sub> * m * i, where K<sub>b</sub> is the ebullioscopic constant of the solvent, m is the molality of the solution, and i is the Van't Hoff factor.
-
Freezing Point Depression: ΔT<sub>f</sub> = K<sub>f</sub> * m * i, where K<sub>f</sub> is the cryoscopic constant of the solvent.
-
Osmotic Pressure: π = iMRT, where π is the osmotic pressure, M is the molarity of the solution, R is the ideal gas constant, and T is the absolute temperature.
These equations are valuable tools in various applications:
-
Antifreeze Solutions: Lowering the freezing point of water using antifreeze solutions (like ethylene glycol) is based on freezing point depression.
-
Desalination: Reverse osmosis utilizes osmotic pressure to remove salt from seawater.
-
Determining Molar Mass: Measurement of colligative properties can be used to determine the molar mass of an unknown solute.
-
Biological Systems: Osmotic pressure is vital in maintaining fluid balance in living organisms.
Advanced Considerations: Beyond Ideal Solutions
The equations presented above assume ideal solutions, where solute-solute, solute-solvent, and solvent-solvent interactions are all equal. However, in real-world scenarios, deviations from ideality occur due to strong intermolecular forces. These deviations lead to activity coefficients which modify the equations to better reflect the behavior of real solutions.
Conclusion: A Holistic Perspective
Colligative properties are a powerful concept illustrating the relationship between the number of solute particles and the physical properties of a solution. While the number of particles is the dominant factor, understanding the role of concentration, dissociation, association, solvent nature, temperature, and deviations from ideal behavior provides a complete understanding of these properties. This knowledge is essential in various scientific fields, from chemistry and biology to engineering and medicine, offering practical insights into diverse applications. By appreciating the intricate interplay of these factors, we can effectively predict and manipulate the behavior of solutions, enabling advancements in technology and a deeper understanding of natural processes.
Latest Posts
Latest Posts
-
What Does The Nernst Equation Tell Us
Mar 30, 2025
-
What Are The Characteristics Of A Solid
Mar 30, 2025
-
What Is The Difference Between Empirical Formula And Molecular Formula
Mar 30, 2025
-
What Is The Function Of A State
Mar 30, 2025
-
How Are Vestigial Structures An Example Of Evidence Of Evolution
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about What Do Colligative Properties Depend On . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
