1s 2 2s 2 2p 6 3s 2 3p 5
Muz Play
Mar 20, 2025 · 5 min read
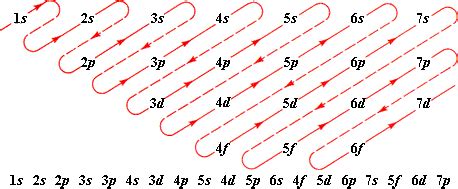
Table of Contents
Decoding 1s² 2s² 2p⁶ 3s² 3p⁵: Unveiling the Secrets of Chlorine's Electronic Structure
The seemingly simple string of numbers and letters, "1s² 2s² 2p⁶ 3s² 3p⁵," holds the key to understanding the fundamental properties of chlorine. This article delves deep into the meaning of this electronic configuration, exploring its implications for chlorine's reactivity, bonding behavior, and position within the periodic table. We'll unravel the complexities of electron shells, subshells, and orbitals, providing a comprehensive understanding of this crucial aspect of atomic structure.
Understanding Electronic Configuration: The Foundation of Atomic Behavior
Electronic configuration describes the arrangement of electrons within an atom's energy levels. This arrangement dictates how an atom interacts with other atoms, forming molecules and exhibiting various chemical and physical properties. The notation uses numbers and letters to represent the different energy levels (shells) and sublevels (subshells) within the atom.
-
Principal Quantum Number (n): The number preceding the letter (e.g., 1, 2, 3) represents the principal energy level or shell. Higher numbers indicate higher energy levels and greater distance from the nucleus.
-
Azimuthal Quantum Number (l): The letter (s, p, d, f) indicates the subshell within a principal energy level. Each subshell has a specific shape and can hold a certain number of electrons:
- s subshell (l=0): Spherical shape, holds up to 2 electrons.
- p subshell (l=1): Dumbbell shape, holds up to 6 electrons (3 orbitals, each holding 2 electrons).
- d subshell (l=2): More complex shape, holds up to 10 electrons.
- f subshell (l=3): Even more complex shape, holds up to 14 electrons.
-
Superscript: The superscript number (e.g.,², ⁶) indicates the number of electrons occupying that particular subshell.
Dissecting the Electronic Configuration of Chlorine (1s² 2s² 2p⁶ 3s² 3p⁵)
Let's break down the electronic configuration of chlorine, 1s² 2s² 2p⁶ 3s² 3p⁵, step-by-step:
-
1s²: This indicates two electrons in the first principal energy level (n=1) and the s subshell. This is the lowest energy level and closest to the nucleus.
-
2s²: Two electrons occupy the s subshell of the second principal energy level (n=2).
-
2p⁶: Six electrons fill the p subshell of the second principal energy level. Remember, the p subshell has three orbitals, each capable of holding two electrons.
-
3s²: Two electrons reside in the s subshell of the third principal energy level (n=3).
-
3p⁵: Five electrons occupy the p subshell of the third principal energy level. This leaves one orbital in the 3p subshell unoccupied.
Implications of Chlorine's Electronic Configuration: Reactivity and Bonding
Chlorine's electronic configuration, particularly the partially filled 3p subshell, is crucial in determining its chemical behavior. The presence of only five electrons in the 3p subshell means it needs one more electron to achieve a stable, filled octet (eight electrons in its outermost shell). This strong tendency to gain an electron makes chlorine highly reactive and readily forms negative ions (anions) with a -1 charge (Cl⁻).
Ionic Bonding: The Drive for Stability
Chlorine readily participates in ionic bonding, a type of chemical bond formed through the electrostatic attraction between oppositely charged ions. Chlorine achieves a stable octet by gaining an electron from a less electronegative atom, typically an alkali metal or alkaline earth metal. For example, in the formation of sodium chloride (NaCl, common table salt), sodium (Na) readily donates an electron to chlorine, forming Na⁺ and Cl⁻ ions, which are then held together by strong electrostatic forces.
Covalent Bonding: Sharing Electrons
While ionic bonding is prevalent for chlorine, it can also participate in covalent bonding, where atoms share electrons to achieve a stable electronic configuration. In covalent bonds, chlorine shares an electron pair with another atom, often another nonmetal. For instance, in chlorine gas (Cl₂), two chlorine atoms share one electron pair, forming a single covalent bond and completing their octets.
Chlorine's Place in the Periodic Table: Group 17 (Halogens)
Chlorine's electronic configuration places it firmly in Group 17 of the periodic table, also known as the halogens. Halogens are characterized by their high electronegativity, meaning they have a strong tendency to attract electrons. This is a direct consequence of their electronic configuration, with one electron short of a complete octet in their outermost shell. This shared characteristic leads to similar chemical properties among halogens, including high reactivity and the formation of salts with metals.
Beyond the Basics: Exploring Further Concepts
The electronic configuration of chlorine opens the door to understanding more advanced concepts in chemistry:
Electron Affinity
Chlorine exhibits a high electron affinity, meaning it releases a significant amount of energy when it gains an electron. This high electron affinity further supports its tendency to form anions and participate in ionic bonding.
Ionization Energy
While chlorine readily gains an electron, removing an electron requires considerable energy. Chlorine's ionization energy is relatively high, reflecting the stability achieved by having a complete octet.
Electronegativity
As mentioned, chlorine has high electronegativity. This property reflects its ability to attract electrons within a chemical bond, contributing to the polarity of many chlorine-containing compounds.
Oxidation States
Chlorine can exhibit various oxidation states, reflecting its ability to gain or lose electrons in different chemical environments. While -1 is the most common oxidation state, it can also exhibit positive oxidation states in some compounds.
Conclusion: The Significance of 1s² 2s² 2p⁶ 3s² 3p⁵
The seemingly simple notation, 1s² 2s² 2p⁶ 3s² 3p⁵, reveals a wealth of information about chlorine's atomic structure and chemical behavior. Understanding this electronic configuration allows us to predict its reactivity, bonding patterns, and position within the periodic table. This knowledge forms the bedrock for understanding the properties of numerous chlorine-containing compounds and their crucial roles in various industrial processes and biological systems. From the humble table salt to more complex industrial chemicals, the influence of chlorine's electronic configuration is widespread and profoundly significant. Further exploration into this configuration and related concepts unlocks a deeper appreciation of the fundamental principles governing the world around us. The seemingly simple string of numbers and letters represents a complex interplay of forces that dictate the behaviour of this vital element.
Latest Posts
Latest Posts
-
What Is Free Energy In Biology
Mar 20, 2025
-
Efield Due To A Point Charge
Mar 20, 2025
-
The Longitudinal Growth Of Long Bones Ceases When
Mar 20, 2025
-
Similar Structures That Evolved Independently Are Called
Mar 20, 2025
-
Two Isotopes Of An Element Differ Only In Their
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about 1s 2 2s 2 2p 6 3s 2 3p 5 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
