Two Isotopes Of An Element Differ Only In Their
Muz Play
Mar 20, 2025 · 6 min read
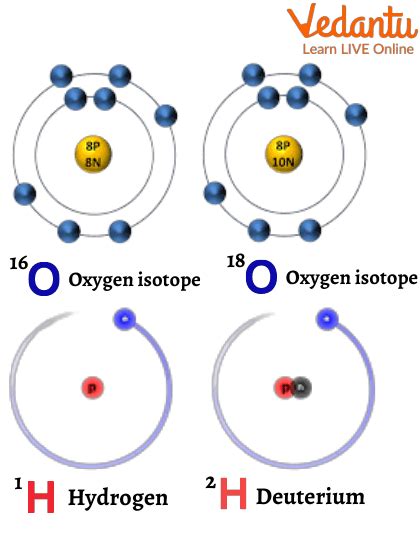
Table of Contents
Two Isotopes of an Element Differ Only in Their Number of Neutrons
Isotopes are variations of a chemical element that share the same number of protons but differ in the number of neutrons within their atomic nuclei. This seemingly small difference has profound implications for the element's properties, impacting everything from its stability to its applications in various fields. Let's delve deeper into understanding this fundamental distinction.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before we explore the nuances of isotopes, it's crucial to grasp the basic building blocks of an atom. An atom comprises three primary subatomic particles:
-
Protons: Positively charged particles residing in the atom's nucleus. The number of protons defines the element's atomic number and determines its identity on the periodic table. For example, all carbon atoms have six protons.
-
Neutrons: Neutrally charged particles also found within the nucleus. Unlike protons, the number of neutrons can vary within the same element, leading to the formation of isotopes.
-
Electrons: Negatively charged particles that orbit the nucleus in shells or energy levels. The number of electrons generally equals the number of protons in a neutral atom. Electrons determine the chemical behavior of an element.
What Makes Isotopes Different? The Neutron Count
The key distinction between isotopes of the same element lies solely in their neutron count. The atomic number, determined by the number of protons, remains constant. However, the mass number, which is the sum of protons and neutrons, differs between isotopes.
For example, consider carbon:
-
Carbon-12 (¹²C): This is the most abundant isotope of carbon, containing six protons and six neutrons (mass number = 12).
-
Carbon-14 (¹⁴C): This is a radioactive isotope of carbon, possessing six protons and eight neutrons (mass number = 14).
Notice that both ¹²C and ¹⁴C are carbon atoms because they both have six protons. The difference in their neutron number accounts for their different mass numbers and crucial differences in their properties.
Isotope Notation: A Clear Representation
Isotopes are commonly represented using a specific notation that clearly conveys their identity:
-
Element Symbol: The standard chemical symbol for the element (e.g., C for carbon, U for uranium).
-
Mass Number (A): The superscript number representing the total number of protons and neutrons (e.g., ¹⁴ in ¹⁴C).
-
Atomic Number (Z): The subscript number representing the number of protons (though often omitted as it's readily identifiable from the element symbol).
This notation efficiently communicates the isotopic composition of an atom.
The Impact of Neutron Number on Isotope Properties
While isotopes of the same element exhibit similar chemical properties due to their identical electron configurations, the differing neutron numbers significantly influence their physical properties:
-
Mass: The most obvious difference is their mass. Isotopes with more neutrons have a higher mass than those with fewer neutrons. This mass difference is crucial in various applications, such as isotopic separation.
-
Nuclear Stability: The neutron-to-proton ratio within the nucleus plays a critical role in determining nuclear stability. Certain neutron-to-proton ratios lead to stable isotopes, while others result in unstable or radioactive isotopes that undergo radioactive decay. This decay can involve the emission of particles like alpha, beta, and gamma radiation.
-
Nuclear Reactions: Radioactive isotopes are instrumental in various nuclear reactions. Their instability enables their use as tracers in biological and environmental studies, as well as in medical imaging and therapy.
-
Density: Although subtle, the difference in mass can lead to slightly different densities for isotopes of the same element. This is particularly noticeable in elements with a wider range of naturally occurring isotopes.
Applications of Isotopes Across Diverse Fields
Isotopes, particularly radioactive isotopes, find extensive application in numerous scientific and technological domains:
1. Medicine: Diagnosis and Treatment
-
Radioactive tracers: Radioactive isotopes, like technetium-99m, are used in medical imaging techniques such as single-photon emission computed tomography (SPECT) and positron emission tomography (PET). These tracers allow doctors to visualize internal organs and detect abnormalities.
-
Radiotherapy: Radioactive isotopes are employed in cancer treatment to target and destroy cancerous cells. For example, iodine-131 is used in thyroid cancer treatment.
2. Environmental Science: Tracing and Monitoring
-
Carbon-14 dating: The radioactive decay of carbon-14 is used to determine the age of organic materials, providing valuable insights into archaeology, paleontology, and climate studies.
-
Water tracing: Radioactive isotopes like tritium are used to trace the movement of water in hydrological systems, allowing scientists to understand groundwater flow and contamination patterns.
3. Industrial Applications: Gauging and Testing
-
Gauging thickness: Radioactive isotopes are used in industrial processes to gauge the thickness of materials like paper and metal sheets.
-
Leak detection: Radioactive tracers can help detect leaks in pipelines and other industrial systems.
4. Scientific Research: Studying Chemical Reactions and Processes
-
Isotopic labeling: Isotopes can be used to label molecules in chemical reactions, allowing scientists to track the movement of atoms and understand reaction mechanisms.
-
Nuclear magnetic resonance (NMR): NMR spectroscopy utilizes the magnetic properties of certain atomic nuclei to obtain detailed information about molecular structure and dynamics. Different isotopes can exhibit different NMR responses.
Isotope Separation: Challenges and Techniques
Separating isotopes is a challenging process, particularly for isotopes with similar masses. Several techniques are employed depending on the isotopes involved and the scale of separation required:
-
Gaseous diffusion: This method exploits the slight difference in diffusion rates of gases containing different isotopes. It is primarily used for uranium isotope separation.
-
Centrifugation: This technique utilizes high-speed centrifuges to separate isotopes based on their mass differences. It is also extensively used in uranium enrichment.
-
Laser isotope separation: This advanced method uses lasers to selectively excite and ionize specific isotopes, facilitating their separation. It offers higher efficiency and selectivity than traditional methods.
Isotopes and Nuclear Energy
The use of isotopes is paramount in nuclear energy production. Specifically, the controlled fission of uranium-235 is utilized in nuclear reactors to generate electricity. The separation of uranium-235 from the more abundant uranium-238 is a critical step in nuclear fuel production.
Conclusion: The Significance of Isotopic Variation
The seemingly minor difference in neutron number between isotopes of the same element has far-reaching consequences. The unique properties of isotopes, particularly their radioactive nature, have revolutionized various fields, from medicine and environmental science to industry and scientific research. Further research and technological advancements continue to expand the applications of isotopes and deepen our understanding of the atom and its behavior. The study of isotopes is an ongoing and critical area of scientific inquiry, constantly revealing new possibilities and impacting our world in profound ways. The precise knowledge of isotope numbers is essential for numerous scientific endeavors, highlighting their central role in various fields of study and application. Understanding the subtle yet significant differences between isotopes enhances our ability to leverage their unique properties for the benefit of humanity.
Latest Posts
Latest Posts
-
What Does Amu Stand For In Chemistry
Mar 21, 2025
-
What Is The Si Unit For Weight
Mar 21, 2025
-
Is Water Boiling A Physical Change
Mar 21, 2025
-
What Is A Point Charge In Physics
Mar 21, 2025
-
Una Maestra A Los Alumnos
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Two Isotopes Of An Element Differ Only In Their . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
