What Does Amu Stand For In Chemistry
Muz Play
Mar 21, 2025 · 6 min read
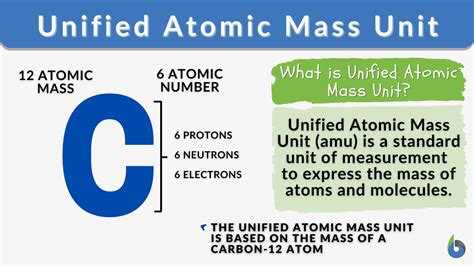
Table of Contents
What Does AMU Stand For in Chemistry? A Deep Dive into Atomic Mass Units
Atomic Mass Unit (amu), also known as Dalton (Da), is a fundamental unit in chemistry and physics used to express the mass of atoms and molecules. Understanding AMU is crucial for grasping concepts like molar mass, stoichiometry, and isotopic abundance. This comprehensive guide delves into the meaning of AMU, its historical context, its applications, and its relationship to other crucial units in chemistry.
Understanding Atomic Mass Units (AMU)
The atomic mass unit (amu) is defined as one twelfth (1/12) the mass of a single unbound neutral atom of carbon-12 (¹²C) in its nuclear and electronic ground state. This means we use the mass of a specific carbon isotope as a reference point for measuring the mass of all other atoms and molecules. Choosing carbon-12 as the standard was a deliberate decision, driven by its abundance and relative ease of measurement. Before this standardized definition, various scales were used, leading to inconsistencies and confusion in scientific literature.
Why Carbon-12?
The selection of carbon-12 as the basis for the AMU scale was not arbitrary. Several factors contributed to its choice:
- Abundance: Carbon-12 is a relatively abundant isotope of carbon, making it readily available for measurements.
- Stability: It's a stable isotope, ensuring consistent and reliable mass measurements.
- Measurability: Its mass can be accurately determined using mass spectrometry techniques.
Using a specific isotope like ¹²C eliminates ambiguity and provides a universally accepted standard for atomic mass.
AMU vs. Dalton (Da)
While the terms AMU and Dalton (Da) are frequently used interchangeably, there's a subtle distinction. AMU is the older term, while Dalton (Da) is the more modern and officially recommended IUPAC (International Union of Pure and Applied Chemistry) unit. However, both units represent the same physical quantity: one-twelfth the mass of a carbon-12 atom. In practice, most scientists use both terms without causing any confusion.
Calculating Atomic Mass Using AMU
Atomic mass, also known as atomic weight, represents the average mass of all isotopes of an element, weighted by their relative abundance in nature. This differs from the mass number, which simply represents the sum of protons and neutrons in a single isotope's nucleus.
The atomic mass is calculated using the following formula:
Atomic Mass = Σ (Mass of Isotope * Fractional Abundance of Isotope)
Where:
- Σ represents the sum of all isotopes.
- Mass of Isotope is the mass of a specific isotope in AMU.
- Fractional Abundance of Isotope is the proportion of that isotope in the naturally occurring element.
Example: Chlorine has two naturally occurring isotopes: ³⁵Cl (75.77% abundance) and ³⁷Cl (24.23% abundance). The mass of ³⁵Cl is approximately 34.97 amu, and the mass of ³⁷Cl is approximately 36.97 amu. To calculate the atomic mass of chlorine:
Atomic Mass of Chlorine = (34.97 amu * 0.7577) + (36.97 amu * 0.2423) ≈ 35.45 amu
This calculated atomic mass (approximately 35.45 amu) is what's typically found on the periodic table for chlorine. It reflects the average mass of chlorine atoms as they occur in nature.
Applications of AMU in Chemistry
AMU finds extensive applications across various branches of chemistry:
1. Stoichiometry and Chemical Calculations:
AMU is fundamental in stoichiometric calculations, which involve determining the amounts of reactants and products in chemical reactions. Molar mass, expressed in grams per mole (g/mol), is directly related to AMU. One mole of a substance contains Avogadro's number (6.022 x 10²³) of entities (atoms, molecules, ions). The molar mass is numerically equal to the atomic mass (in AMU) of the element or the sum of the atomic masses of the atoms in a molecule.
2. Mass Spectrometry:
Mass spectrometry is an analytical technique that measures the mass-to-charge ratio of ions. The results are often expressed in AMU, allowing for the identification of molecules and the determination of their isotopic composition.
3. Nuclear Chemistry:
In nuclear chemistry, understanding the masses of isotopes in AMU is crucial for calculating energy changes during nuclear reactions. The mass defect, the difference between the mass of the nucleus and the sum of the masses of its constituent protons and neutrons, is directly related to the binding energy of the nucleus, calculated using Einstein's famous equation, E=mc².
4. Biochemistry and Molecular Biology:
AMU plays a vital role in biochemistry and molecular biology. The masses of proteins, peptides, and other biomolecules are expressed in AMU or Da, facilitating the analysis of their composition and structure using techniques such as mass spectrometry. Understanding the mass of a protein is critical for studying protein folding, interactions, and function.
5. Material Science:
In material science, AMU helps characterize materials at the atomic and molecular level. It's especially relevant when studying alloys, polymers, and nanomaterials, where precise knowledge of the masses of the constituent atoms is needed to understand their properties and behavior.
AMU and Other Units: A Comparative Look
AMU is intrinsically linked to other units in chemistry:
-
Gram (g): One gram is approximately 6.022 x 10²³ AMU. This relationship is directly related to Avogadro's number and is crucial for converting between AMU and grams.
-
Mole (mol): A mole is the amount of substance that contains Avogadro's number of entities. The molar mass of a substance (g/mol) is numerically equivalent to its mass in AMU.
-
Electronvolt (eV): Electronvolt, a unit of energy, is sometimes used in nuclear and atomic physics to represent the binding energy of nucleons within the nucleus. The conversion factor between AMU and eV can be used to relate mass and energy.
Beyond the Basics: Isotopes and Isotopic Abundance
The concept of isotopes is inextricably linked to AMU. Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons. This results in variations in their atomic mass. For example, carbon has two main isotopes: ¹²C (carbon-12) and ¹³C (carbon-13). ¹²C has a mass of approximately 12 amu, while ¹³C has a mass of approximately 13 amu. The relative abundance of these isotopes in nature dictates the average atomic mass of carbon as reported on the periodic table.
Understanding isotopic abundance is essential for accurate calculations of atomic mass and for interpreting mass spectrometry data. Variations in isotopic abundance can provide insights into geological processes, environmental factors, and even forensic investigations.
Conclusion: The Enduring Importance of AMU
The atomic mass unit (amu) or Dalton (Da) is a cornerstone unit in chemistry and related fields. Its role in understanding atomic and molecular masses, stoichiometry, and various analytical techniques is undeniable. From calculating the molar masses of compounds to interpreting mass spectrometry data and exploring nuclear processes, AMU provides a fundamental framework for comprehending the composition and behavior of matter at the atomic and molecular level. While the definition has evolved over time, its significance remains steadfast, ensuring accurate and consistent measurements across diverse scientific disciplines. The continuing relevance of AMU underscores its enduring importance in the ongoing quest to unravel the intricacies of the chemical world.
Latest Posts
Latest Posts
-
Mixing An Acid And A Base
Mar 28, 2025
-
Difference Between Meiosis 1 And Meiosis 2
Mar 28, 2025
-
How To Find The Hydronium Ion Concentration
Mar 28, 2025
-
What Is The Mass Of A Proton In Amu
Mar 28, 2025
-
During The Secretory Phase Of The Uterine Cycle
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about What Does Amu Stand For In Chemistry . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
