A Bunch Of Amino Acids Attached Together Is Called A
Muz Play
Mar 24, 2025 · 6 min read
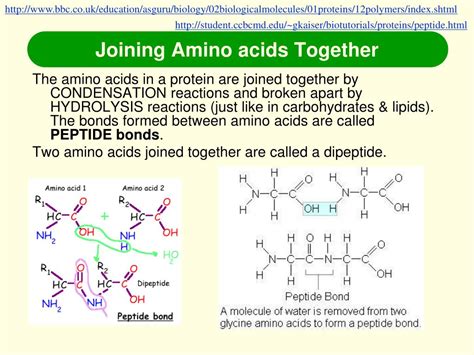
Table of Contents
A Bunch of Amino Acids Attached Together is Called a: Exploring the World of Peptides and Proteins
A bunch of amino acids attached together is called a peptide or, if it's a long chain with a specific three-dimensional structure, a protein. Understanding this fundamental concept is crucial to grasping the complexities of biochemistry and its role in all living organisms. This article delves deep into the fascinating world of amino acids, peptides, and proteins, exploring their structure, function, and importance in various biological processes.
What are Amino Acids?
Before understanding peptides and proteins, we must first understand their building blocks: amino acids. These are organic molecules containing both an amino group (-NH2) and a carboxyl group (-COOH) attached to the same carbon atom, known as the α-carbon. The α-carbon also has a hydrogen atom and a side chain (R group) attached. It's this R group that differs between the 20 standard amino acids, giving each its unique properties.
The 20 Standard Amino Acids: A Diverse Cast of Characters
The 20 standard amino acids are categorized based on their R group's properties:
-
Nonpolar, aliphatic amino acids: These have hydrophobic (water-repelling) side chains. Examples include glycine, alanine, valine, leucine, isoleucine, and methionine. These amino acids often contribute to the hydrophobic core of proteins.
-
Aromatic amino acids: These have ring structures in their side chains, making them relatively hydrophobic. Examples include phenylalanine, tyrosine, and tryptophan. These amino acids can absorb UV light, a property utilized in protein analysis techniques.
-
Polar, uncharged amino acids: These have hydrophilic (water-attracting) side chains. Examples include serine, threonine, cysteine, asparagine, and glutamine. They often participate in hydrogen bonding within proteins and with water molecules.
-
Positively charged (basic) amino acids: These have side chains with positive charges at physiological pH. Examples include lysine, arginine, and histidine. They often interact with negatively charged molecules.
-
Negatively charged (acidic) amino acids: These have side chains with negative charges at physiological pH. Examples include aspartic acid and glutamic acid. They also participate in ionic interactions.
The unique properties of each amino acid's R group contribute significantly to the overall properties and functions of the resulting peptides and proteins.
Peptide Bonds: Linking Amino Acids Together
Amino acids are joined together by peptide bonds, a type of covalent bond. This bond forms between the carboxyl group (-COOH) of one amino acid and the amino group (-NH2) of another, releasing a molecule of water (H2O) in a process called dehydration synthesis or condensation. The resulting molecule is a dipeptide if it contains two amino acids, a tripeptide if it contains three, and so on.
Longer chains of amino acids linked by peptide bonds are called polypeptides. The sequence of amino acids in a polypeptide chain is called its primary structure. This sequence dictates all subsequent levels of protein structure.
Levels of Protein Structure: From Linear Chain to 3D Marvel
Proteins exhibit a hierarchical structure, characterized by four levels of organization:
1. Primary Structure: The Amino Acid Sequence
As mentioned earlier, the primary structure is the linear sequence of amino acids in a polypeptide chain. This sequence is determined by the genetic code, and even a single amino acid change can significantly affect the protein's function. This is exemplified by sickle cell anemia, caused by a single amino acid substitution in the hemoglobin protein.
2. Secondary Structure: Local Folding Patterns
The primary structure folds into regular, repeating patterns called secondary structures. These patterns are stabilized by hydrogen bonds between the peptide backbone atoms. Common secondary structures include:
-
α-helices: A right-handed coiled structure stabilized by hydrogen bonds between every fourth amino acid.
-
β-sheets: Extended polypeptide chains arranged side-by-side, stabilized by hydrogen bonds between adjacent strands. These can be parallel (strands running in the same direction) or antiparallel (strands running in opposite directions).
-
Turns and loops: Short, irregular segments that connect α-helices and β-sheets.
3. Tertiary Structure: The Overall 3D Shape
The tertiary structure refers to the overall three-dimensional arrangement of a polypeptide chain, including all its secondary structures. This structure is stabilized by various interactions between the R groups of amino acids, including:
-
Hydrophobic interactions: Nonpolar side chains cluster together in the protein's interior, away from water.
-
Hydrogen bonds: Polar side chains form hydrogen bonds with each other or with water molecules.
-
Ionic bonds (salt bridges): Positively and negatively charged side chains attract each other.
-
Disulfide bonds: Covalent bonds between cysteine residues, forming strong cross-links within the protein.
The tertiary structure determines the protein's function. A change in tertiary structure, often caused by denaturation (unfolding), can lead to loss of function.
4. Quaternary Structure: Multiple Polypeptide Chains
Some proteins are composed of multiple polypeptide chains, each with its own tertiary structure. The arrangement of these chains relative to each other is called the quaternary structure. This structure is also stabilized by various interactions, similar to those involved in tertiary structure. Hemoglobin, for example, has a quaternary structure consisting of four polypeptide chains.
Functions of Peptides and Proteins: A Multitude of Roles
Peptides and proteins play a vast array of roles in living organisms, including:
-
Enzymes: Catalyze biochemical reactions. Examples include digestive enzymes like amylase and protease.
-
Structural proteins: Provide structural support. Examples include collagen (in connective tissue) and keratin (in hair and nails).
-
Transport proteins: Carry molecules across membranes or throughout the body. Examples include hemoglobin (carrying oxygen) and serum albumin (carrying fatty acids).
-
Hormones: Act as chemical messengers. Examples include insulin (regulating blood sugar) and glucagon (raising blood sugar).
-
Antibodies: Part of the immune system, defending against foreign invaders.
-
Receptor proteins: Bind to specific molecules, triggering cellular responses.
-
Motor proteins: Enable movement within cells or of the entire organism. Examples include myosin (in muscle contraction) and kinesin (in intracellular transport).
Peptide vs. Protein: A Key Distinction
While the terms are often used interchangeably, there's a subtle yet important difference between peptides and proteins. Generally, a peptide is considered a shorter chain of amino acids, typically less than 50 amino acids, while a protein is a longer chain, typically more than 50 amino acids, with a well-defined three-dimensional structure that is crucial for its biological function. However, the line between peptide and protein can sometimes be blurry, and the terms are often used contextually. Many biologically active peptides, for example, exhibit highly specific functions despite their relatively small size.
The Importance of Protein Folding and Misfolding
The proper folding of proteins is essential for their function. Errors in protein folding can lead to the formation of misfolded proteins, which can accumulate and cause various diseases, including:
-
Alzheimer's disease: Amyloid plaques composed of misfolded proteins.
-
Parkinson's disease: Lewy bodies containing misfolded α-synuclein.
-
Mad cow disease (Bovine spongiform encephalopathy): Prion diseases caused by misfolded prion proteins.
-
Cystic fibrosis: Caused by misfolded cystic fibrosis transmembrane conductance regulator (CFTR) protein.
Conclusion: A World of Amino Acid Combinations
From the simplest dipeptides to the complex macromolecules that drive life's processes, the linkage of amino acids through peptide bonds forms the foundation of a vast and intricate world. Understanding the structure and function of peptides and proteins is paramount in diverse fields, from medicine and pharmaceuticals to biotechnology and materials science. The continuous research in this field promises further insights into the remarkable versatility and importance of these essential molecules. Continued exploration of protein folding mechanisms and the development of therapies targeting misfolded proteins offer hope for treating a wide range of debilitating diseases. The seemingly simple act of connecting amino acids creates a universe of complexity and potential.
Latest Posts
Latest Posts
-
Activity Series Of Metals And Non Metals
Mar 28, 2025
-
How Many Unpaired Electrons Does Carbon Have
Mar 28, 2025
-
What Is Damping In A Wave
Mar 28, 2025
-
How To Calculate Heat Of Dissolution Without Temperature
Mar 28, 2025
-
Which Part Of The Brain Contains The Cardiac Control Center
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about A Bunch Of Amino Acids Attached Together Is Called A . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
