A Mixture In Which The Composition Is Uniform Throughout
Muz Play
Mar 16, 2025 · 6 min read
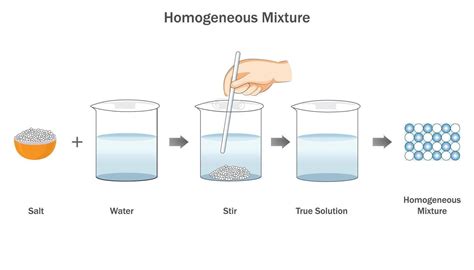
Table of Contents
A Mixture in Which the Composition is Uniform Throughout: A Deep Dive into Homogeneous Mixtures
A mixture is a substance comprising two or more components not chemically bonded. A key characteristic differentiating mixtures is the uniformity of their composition. When the composition is uniform throughout, meaning the components are evenly distributed at a microscopic level, we call it a homogeneous mixture. This contrasts with a heterogeneous mixture, where the composition is non-uniform and different parts have different properties. This article delves into the fascinating world of homogeneous mixtures, exploring their characteristics, examples, properties, and significance in various fields.
Understanding Homogeneous Mixtures: A Microscopic Perspective
At the heart of defining a homogeneous mixture lies the concept of uniformity. Imagine zooming in on a sample of a homogeneous mixture using a powerful microscope. No matter where you look, the composition remains consistent. You won't see distinct clusters or regions of different components. The components are intimately mixed at a molecular or ionic level, resulting in a single phase. This uniformity is what sets homogeneous mixtures apart and makes them appear visually consistent throughout.
Key Characteristics of Homogeneous Mixtures
- Uniform Composition: This is the defining characteristic. The ratio of components remains the same throughout the mixture.
- Single Phase: Homogeneous mixtures exist in a single phase—solid, liquid, or gas. You won't observe distinct layers or boundaries between different components.
- Invisible Components: In many cases, the individual components are not visibly distinguishable, even with magnification. They're so thoroughly mixed that they appear as a single substance.
- Filtration Difficulty: Separating components of a homogeneous mixture requires specialized techniques like distillation or chromatography because simple filtration won't work.
Examples of Homogeneous Mixtures: From Everyday Life to Advanced Applications
Homogeneous mixtures are ubiquitous, encountered in numerous everyday situations and advanced scientific applications. Let's explore some common examples categorized by their phase:
Gaseous Homogeneous Mixtures: The Air We Breathe
Air is a classic example of a gaseous homogeneous mixture. It's a blend of primarily nitrogen, oxygen, argon, carbon dioxide, and trace amounts of other gases. These gases are evenly dispersed, making air appear uniform throughout. The ratio of these gases might vary slightly depending on location and altitude, but it's generally consistent enough to be considered homogeneous.
Liquid Homogeneous Mixtures: Solutions and Alloys
Many common liquids are homogeneous mixtures. Solutions, formed by dissolving a solute in a solvent, are excellent examples. Saltwater is a simple solution where salt (solute) is dissolved in water (solvent). The salt molecules are evenly distributed throughout the water, resulting in a uniform composition. Other examples include sugar dissolved in water, alcohol in water, and many commercially available beverages.
Liquid homogeneous mixtures also extend to metallic alloys. Alloys are mixtures of two or more metals, often possessing properties superior to the individual metals. Brass (copper and zinc) and bronze (copper and tin) are classic examples. The metals are melted together and then cooled, creating a uniform solid solution.
Solid Homogeneous Mixtures: A World of Alloys and More
While less intuitively apparent, many solid materials are homogeneous mixtures. Beyond metallic alloys mentioned above, numerous solid solutions exist. Certain types of glass are homogeneous mixtures of silica (silicon dioxide) and other metal oxides. The components are evenly distributed throughout the solid structure, resulting in a uniform appearance and properties. Some plastics also fall into this category, with various additives uniformly dispersed within the polymer matrix.
Properties of Homogeneous Mixtures: Composition and Behavior
The properties of a homogeneous mixture are dictated by the properties of its components and their relative proportions. However, it's crucial to understand that the properties of a homogeneous mixture are not simply the average of the individual component properties. Interactions between components often lead to emergent properties that are distinct from those of the individual constituents.
For instance, consider the boiling point of a saltwater solution. The boiling point of the solution is higher than the boiling point of pure water. This elevation in boiling point is a colligative property, depending on the concentration of solute particles in the solution. Similarly, the freezing point of the solution is lower than that of pure water—a phenomenon known as freezing point depression.
Separating Components of Homogeneous Mixtures: Advanced Techniques
Separating components of a homogeneous mixture requires techniques that exploit differences in physical properties such as boiling point, solubility, or adsorption. These techniques include:
- Distillation: This process separates liquids with different boiling points. A mixture is heated, and the component with the lower boiling point vaporizes first, then condenses separately.
- Chromatography: This technique separates components based on their differing affinities for a stationary phase and a mobile phase. Components move at different rates, allowing for separation.
- Crystallization: This process exploits differences in solubility to separate components. A solution is slowly cooled or solvent is evaporated, causing the least soluble component to crystallize first.
- Evaporation: This simple technique can be used to separate a dissolved solid from a liquid solvent. The solvent evaporates, leaving behind the solid.
Significance of Homogeneous Mixtures: Applications Across Diverse Fields
Homogeneous mixtures play a crucial role in various fields:
Medicine and Pharmaceuticals: Precise Dosage and Delivery
Many pharmaceutical formulations are homogeneous mixtures. Solutions, suspensions, and emulsions are used to deliver precise dosages of drugs. The uniform distribution of the active ingredient ensures consistent effectiveness.
Materials Science and Engineering: Designing Novel Materials
Homogeneous mixtures are essential in materials science for creating alloys, polymers, and ceramics with tailored properties. By controlling the composition and processing, engineers can design materials with specific strength, conductivity, or other desired characteristics.
Food Science and Technology: Creating Consistent Products
Homogeneous mixtures are fundamental to food science. Many processed foods, beverages, and condiments are homogeneous mixtures designed for consistency in taste, texture, and appearance.
Environmental Science: Understanding Atmospheric Composition and Pollution
Analyzing the homogeneous mixture of air helps environmental scientists monitor pollution levels and assess the impact of various pollutants on air quality. Understanding the composition of water bodies as homogeneous mixtures is crucial for monitoring water quality and detecting pollutants.
Conclusion: A Foundation of Chemistry and Beyond
Homogeneous mixtures are fundamental to chemistry and have far-reaching implications across diverse scientific and technological fields. Their uniform composition simplifies many analyses and applications, leading to improvements in various products and processes. From the air we breathe to the materials we use daily, homogeneous mixtures are essential components of the world around us. The principles governing their formation, properties, and separation techniques remain areas of ongoing research and development, continuously shaping our understanding of matter and its behavior. Understanding homogeneous mixtures opens doors to a deeper appreciation of the complexities and intricacies of the material world. Continued research in this area promises further advancements in diverse fields, creating innovative solutions to global challenges and improving the quality of life.
Latest Posts
Latest Posts
-
Are Amino Acid Bonds To Water Intermolecular
Mar 16, 2025
-
In Rc Circuit Should Capacitor Be First Or Resistor
Mar 16, 2025
-
Mass Of An Electron In Amu
Mar 16, 2025
-
Graph Of A Second Order Reaction
Mar 16, 2025
-
Experiment 1 The Densities Of Liquids And Solids
Mar 16, 2025
Related Post
Thank you for visiting our website which covers about A Mixture In Which The Composition Is Uniform Throughout . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
