Mass Of An Electron In Amu
Muz Play
Mar 16, 2025 · 6 min read
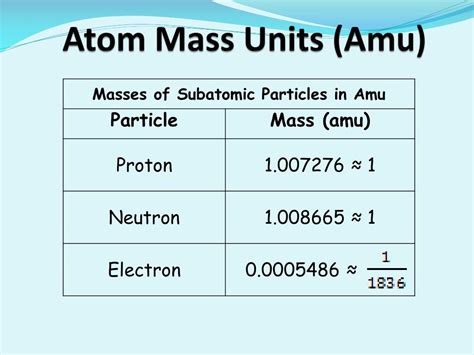
Table of Contents
The Mass of an Electron in AMU: A Deep Dive
The electron, a fundamental subatomic particle, plays a crucial role in the structure and behavior of matter. Understanding its properties, particularly its mass, is essential to grasping various concepts in physics and chemistry. While the electron's mass is often expressed in kilograms, another frequently used unit is the atomic mass unit (amu), also known as the dalton (Da). This article will delve deep into the mass of an electron in amu, exploring its determination, significance, and applications.
Understanding the Atomic Mass Unit (amu)
Before we delve into the electron's mass, let's clarify the atomic mass unit. The amu is a relative unit of mass defined as one-twelfth the mass of a neutral carbon-12 atom. This means that one amu is approximately 1.66053907 × 10⁻²⁷ kilograms. The use of amu is particularly convenient in chemistry and atomic physics because it simplifies the representation of atomic and molecular masses. Instead of dealing with extremely small numbers in kilograms, we can use relatively larger numbers in amu, making calculations easier and more manageable.
Determining the Mass of an Electron in AMU
The precise determination of the electron's mass is a complex process involving sophisticated experimental techniques. Early attempts relied on measuring the charge-to-mass ratio (e/m) of the electron, using techniques such as Thomson's cathode ray tube experiments. While these experiments provided valuable information about the electron's properties, they didn't directly measure its mass. More accurate measurements were made possible through advancements in experimental techniques, including:
1. Millikan's Oil Drop Experiment:
Robert Millikan's oil drop experiment, conducted in the early 20th century, played a pivotal role in determining the elementary charge (e) of the electron. By observing the motion of charged oil droplets in an electric field, Millikan precisely determined the charge of a single electron. Combining this value with the previously determined charge-to-mass ratio (e/m), scientists could then calculate the electron's mass.
2. Advanced Spectroscopic Techniques:
Modern techniques, such as high-precision spectroscopy, significantly improved the accuracy of mass measurements. These techniques involve analyzing the spectral lines emitted or absorbed by atoms and molecules. The precise wavelengths of these spectral lines are sensitive to the masses of the constituent particles, including the electrons. By carefully analyzing these spectra, scientists can determine the electron's mass with extreme precision.
3. Penning Traps:
Penning traps are sophisticated instruments used for trapping charged particles like electrons using a combination of electric and magnetic fields. By precisely measuring the cyclotron frequency of the trapped electron, which is related to its mass and charge, scientists can determine the electron's mass with remarkable accuracy. These traps are capable of achieving extremely high precision, leading to some of the most accurate measurements of the electron's mass.
The Mass of an Electron in AMU: The Value and its Implications
The currently accepted value for the mass of an electron is approximately 5.48579909070 × 10⁻⁴ amu. This incredibly small mass highlights the relatively insignificant contribution of electrons to the overall mass of an atom. The vast majority of an atom's mass is concentrated in its nucleus, which consists of protons and neutrons.
This small mass has profound implications across various scientific fields:
1. Chemistry and Chemical Reactions:
In chemical reactions, the mass of electrons is often neglected because it is significantly smaller compared to the mass of protons and neutrons. This simplification often doesn't significantly affect the accuracy of calculations, making it easier to predict reaction stoichiometry and thermodynamics.
2. Nuclear Physics:
In nuclear physics, however, the electron's mass becomes relevant in processes like beta decay, where a neutron transforms into a proton, an electron (beta particle), and an antineutrino. The mass difference between the neutron and the resulting proton and electron contributes to the energy released during beta decay.
3. Particle Physics:
In particle physics, the precise mass of the electron is a crucial parameter in various theoretical models and calculations involving fundamental interactions. The electron's mass is a fundamental constant, and its value plays a role in understanding the properties of the Standard Model of particle physics.
4. Astrophysics and Cosmology:
The electron's mass is also important in astrophysics and cosmology. The abundance of electrons in stars and other celestial objects affects their overall properties, including their radiative behavior and gravitational interactions. Accurate knowledge of the electron's mass contributes to more precise models of stellar evolution and cosmic processes.
Comparing the Electron's Mass to Other Subatomic Particles
To appreciate the electron's diminutive mass, let's compare it to other subatomic particles:
- Proton: The mass of a proton is approximately 1836 times greater than the mass of an electron.
- Neutron: The mass of a neutron is approximately 1839 times greater than the mass of an electron.
- Muon: The muon, a heavier cousin of the electron, is approximately 207 times more massive.
This significant mass difference highlights the dominance of protons and neutrons in determining the overall mass of an atom.
Applications of the Electron's Mass Knowledge
The precise knowledge of the electron's mass has numerous applications:
- High-precision measurements in various scientific experiments: Accurate determination of the electron's mass serves as a fundamental standard for calibration and validation of measurements in different fields.
- Testing fundamental physics theories: The electron's mass plays a crucial role in testing the validity of theoretical models in physics, particularly in quantum electrodynamics (QED) and the Standard Model.
- Development of advanced technologies: Understanding electron behavior is crucial for developing and improving technologies that rely on electron manipulation, such as electron microscopes, transistors, and particle accelerators.
Future Research and Challenges
Despite the remarkable progress in determining the electron's mass, research continues to refine our understanding. Scientists strive for even greater accuracy in mass measurement, pushing the boundaries of experimental techniques. This ongoing research will help improve our understanding of fundamental physics and contribute to advancements in various technological applications. One ongoing challenge is the search for any potential variations in the electron's mass over time or across different regions of the universe. Such discoveries could have profound implications for our understanding of cosmology and fundamental physics.
Conclusion
The mass of an electron, though exceedingly small, plays a critical role in our understanding of the universe. Expressed in atomic mass units, this mass provides a convenient and relative measure within the realm of atomic and molecular physics and chemistry. Its accurate determination, aided by advancements in experimental techniques, underpins our understanding of various physical phenomena and underpins the advancement of numerous technologies. Further research into the electron's mass, however minute, promises to continue refining our knowledge of the fundamental building blocks of matter and the universe itself. The ongoing quest for ever-greater precision in measuring this seemingly insignificant mass serves as a testament to the relentless pursuit of scientific knowledge and its profound impact on our understanding of the world around us.
Latest Posts
Latest Posts
-
Differential Rate Law For Zero Order Reaction
Mar 17, 2025
-
Cell The Basic Unit Of Life
Mar 17, 2025
-
An Increase In The Aggregate Expenditures Schedule
Mar 17, 2025
-
A Temporary Mixture The Particles Will Eventually Settle
Mar 17, 2025
-
Why Do Ions Travel Back And Forth In Orbitrap
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Mass Of An Electron In Amu . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
